Abstract
Background/Objectives:
Chronic low-grade inflammation/meta-inflammation in adipose tissue leads to obesity-associated metabolic complications. Despite growing understanding, the roles of immune cell subsets, their interrelationship, and chronological events leading to progression of obesity-associated insulin resistance (IR) remains unclear.
Methods:
We carried out temporal immunometabolic profiling of adipose tissue from C57BL/6 mice fed a high-fat diet (HFD) for 4, 8, 12, 16, and 20 weeks. We used clodronate sodium liposomes (CLODs) to deplete macrophages and disodium cromoglycate sodium liposomes (DSCGs) to stabilize mast cells.
Results:
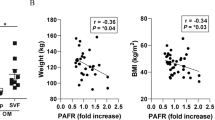
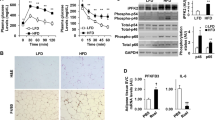
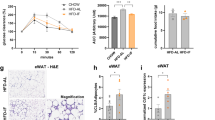
In the temporal HFD settings, mice showed progressive glucose intolerance, insulin resistance, and adipose tissue senescence. Histochemistry analysis of epididymal white adipose tissue (eWAT) using picro-sirius red and Masson’s trichrome staining showed extensive collagen deposition in the 16th and 20th weeks. Flow cytometry analysis of the stromal vascular fraction (SVF) from eWAT revealed T-cell subsets as early-phase components and pro-inflammatory macrophages, as well as mast cells as the later phase components during obesity progression. In our therapeutic strategies, macrophage depletion by CLOD and mast stabilization by DSCG attenuated obesity, adipose tissue fibrosis, and improved whole-body glucose homeostasis. In addition, mast cell stabilization also attenuated senescence (p53 and X-gal staining) in eWAT, signifying the role of mast cells over macrophages during obesity.
Conclusion:
New-generation mast cell stabilizers can be exploited for the treatment of obesity-associated metabolic complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hu FB, Satija A, Manson JE. Curbing the diabetes pandemic: the need for global policy solutions. JAMA. 2015;313:2319–20.
Kanuri BN, Kanshana JS, Rebello SC, Pathak P, Gupta AP, Gayen JR, et al. Altered glucose and lipid homeostasis in liver and adipose tissue pre-dispose inducible NOS knockout mice to insulin resistance. Sci Rep. 2017;7:41009.
Roh E, Song DK, Kim MS. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med. 2016;48:e216.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30.
Lawler HM, Underkofler CM, Kern PA, Erickson C, Bredbeck B, Rasouli N. Adipose tissue hypoxia, inflammation, and fibrosis in obese insulin-sensitive and obese insulin-resistant subjects. J Clin Endocrinol Metab. 2016;101:1422–8.
Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity. 2010;18:1918–25.
Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4 + T cells in mice. Diabetes. 2013;62:2762–72.
Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3:545–56.
Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26:357–66.
Jang JE, Ko MS, Yun JY, Kim MO, Kim JH, Park HS, et al. Nitric oxide produced by macrophages inhibits adipocyte differentiation and promotes profibrogenic responses in preadipocytes to induce adipose tissue fibrosis. Diabetes. 2016;65:2516–28.
Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8.
Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5.
Zand H, Homayounfar R, Cheraghpour M, Jeddi-Tehrani M, Ghorbani A, Pourvali K, et al. Obesity-induced p53 activation in insulin-dependent and independent tissues is inhibited by beta-adrenergic agonist in diet-induced obese rats. Life Sci. 2016;147:103–9.
Kung CP, Leu JI, Basu S, Khaku S, Anokye-Danso F, Liu Q, et al. The P72R polymorphism of p53 predisposes to obesity and metabolic dysfunction. Cell Rep. 2016;14:2413–25.
Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–7.
Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord: J Int Assoc Study Obes. 2000;24:639–46.
Kumar D, Goand UK, Gupta S, Shankar K, Varshney S, Rajan S, et al. Saroglitazar reduces obesity and associated inflammatory consequences in murine adipose tissue. Eur J Pharmacol. 2018;822:32–42.
Luo T, Nocon A, Fry J, Sherban A, Rui X, Jiang B, et al. AMPK activation by metformin suppresses abnormal extracellular matrix remodeling in adipose tissue and ameliorates insulin resistance in obesity. Diabetes. 2016;65:2295–310.
Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50.
Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–9.
Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–21.
Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–54.
Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16:376–85.
Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA. 2010;107:9765–70.
Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–22.
Markar SR, Wahlin K, Lagergren P, Lagergren J. University hospital status and prognosis following surgery for esophageal cancer. Eur J Surg Oncol. 2016;42:1191–5.
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30.
Zhou Y, Yu X, Chen H, Sjoberg S, Roux J, Zhang L, et al. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22:1045–58.
Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, et al. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS ONE. 2011;6:e24358.
Acknowledgements
We are grateful for the excellent instrumental and technical support from institutional SAIF division. This manuscript bears CSIR-CDRI communication number 9735.
Funding
This study was supported by CSIR-CDRI Network project: “Towards holistic understanding of complex diseases: Unraveling the threads of complex disease (THUNDER) Project No: BSC0102. DK, SP, and AS are supported by SRF-CSIR fellowship, New Delhi. KS, SR, and AG are supported by SRF-UGC, New Delhi. SV is supported by SRF-ICMR, New Delhi. SG is supported by DBT-JRF fellowship, New Delhi.
Author contribution
DK and ANG conceptualized the idea. DK performed animal experiments, western blotting, RT-PCR, flow cytometry, and tissue staining. KS performed ELISA assay. SKP and AM developed formulations. AG and SV did metabolic experiments. SR did western blotting in the BAT. AS and SG performed RT-PCR analysis. ALV analyzed FACS data. All the authors participated in drafting and reviewing the manuscript. ANG is the guarantor of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kumar, D., Pandya, S.K., Varshney, S. et al. Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: manifestations of mast cells in fibrosis and senescence. Int J Obes 43, 1281–1294 (2019). https://doi.org/10.1038/s41366-018-0228-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0228-5
This article is cited by
-
Adipose tissue depot specific expression and regulation of fibrosis-related genes and proteins in experimental obesity
Mammalian Genome (2024)
-
Crosstalk Between Mast Cells and Adipocytes in Physiologic and Pathologic Conditions
Clinical Reviews in Allergy & Immunology (2020)
-
Role of innate immune cells in metabolism: from physiology to type 2 diabetes
Seminars in Immunopathology (2019)