Abstract
Background/Objectives
High-fat diet consumption is known to trigger an inflammatory response in the hypothalamus, which has been characterized by an initial expression of pro-inflammatory genes followed by hypothalamic astrocytosis, microgliosis, and the appearance of neuronal injury markers. The specific effects of high-fat diet on hypothalamic energy metabolism and neurotransmission are however not yet known and have not been investigated before.
Subjects/Methods
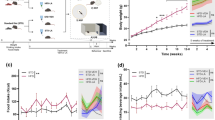
We used 1H and 13C magnetic resonance spectroscopy (MRS) and immunofluorescence techniques to evaluate in vivo the consequences of high-saturated fat diet administration to mice, and explored the effects on hypothalamic metabolism in three mouse cohorts at different time points for up to 4 months.
Results
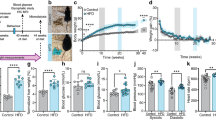
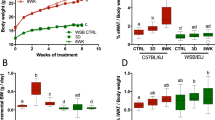
We found that high-fat diet increases significantly the hypothalamic levels of glucose (P < 0.001), osmolytes (P < 0.001), and neurotransmitters (P < 0.05) from 2 months of diet, and alters the rates of metabolic (P < 0.05) and neurotransmission fluxes (P < 0.001), and the contribution of non-glycolytic substrates to hypothalamic metabolism (P < 0.05) after 10 weeks of high-fat feeding.
Conclusions/interpretation
We report changes that reveal a high-fat diet-induced alteration of hypothalamic metabolism and neurotransmission that is quantifiable by 1H and 13C MRS in vivo, and present the first evidence of the extension of the inflammation pathology to a localized metabolic imbalance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
James WP. WHO recognition of the global obesity epidemic. Int J Obes. 2008;32(Suppl 7):S120–6.
Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol Sin. 2012;33:173–81.
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95.
Karnani M, Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am J Physiol Regul Integr Comp Physiol. 2011;300:R47–55.
Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–7.
Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582:132–41.
Soares AF, Duarte JMN, Gruetter R. Increased hepatic fatty acid polyunsaturation precedes ectopic lipid deposition in the liver in adaptation to high-fat diets in mice. Magma. 2017;341–54.
Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–90.
Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA. 2010;107:14875–80.
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–9.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62.
Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26:185–97.e3.
Cai D. One step from prediabetes to diabetes: hypothalamic inflammation? Endocrinology. 2012;153:1010–3.
Dalvi PS, Chalmers JA, Luo V, Han DY, Wellhauser L, Liu Y, et al. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-alpha on appetite-regulating NPY neurons. Int J Obes. 2017;41:149–58.
Duarte JM, Lei H, Mlynarik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage. 2012;61:342–62.
Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Oz G. Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–38.
Lizarbe B, Lei H, Duarte JMN, Lanz B, Cherix A, Gruetter R. Feasibility of in vivo measurement of glucose metabolism in the mouse hypothalamus by (1) H-[(13) C] MRS at 14.1T. Magn Reson Med. 2018;80:874–84.
Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 2008.
Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–23.
Mlynarik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006;56:965–70.
Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–79.
Xin L, Mlynarik V, Lanz B, Frenkel H, Gruetter R. 1H-[13C] NMR spectroscopy of the rat brain during infusion of [2-13C] acetate at 14.1 T. Magn Reson Med. 2010;64:334–40.
Tannus A, Garwood M. Improved performance of frequency-swept pulses using offset-independent adiabaticity. J Magn Reson Ser A. 1996;120:133–7.
Lizarbe B, Cherix A, Gruetter R. In vivo heteronuclear magnetic resonance spectroscopy. Methods Mol Biol. 2018;1718:169–87.
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9.
Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–43.
Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LC Model. NMR Biomed. 2001;14:260–4.
Xin L, Lanz B, Lei H, Gruetter R. Assessment of metabolic fluxes in the mouse brain in vivo using 1H-[13C] NMR spectroscopy at 14.1 T. J Cereb Blood Flow Metab. 2015;35:759–65.
Tkac I, Henry PG, Andersen P, Keene CD, Low WC, Gruetter R. Highly resolved in vivo 1H NMR spectroscopy of the mouse brain at 9.4 T. Magn Reson Med. 2004;52:478–84.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Duarte JM, Lanz B, Gruetter R. Compartmentalized cerebral metabolism of [1,6-(13)C] glucose determined by in vivo (13)C NMR spectroscopy at 14.1 T. Front Neuroenergetics. 2011;3:3.
Lanz B, Gruetter R, Duarte JM. Metabolic flux and compartmentation analysis in the brain in vivo. Front Endocrinol. 2013;4:156.
Sonnay S, Duarte JM, Just N, Gruetter R. Compartmentalised energy metabolism supporting glutamatergic neurotransmission in response to increased activity in the rat cerebral cortex: a 13C MRS study in vivo at 14.1 T. J Cereb Blood Flow Metab. 2016;36:928–40.
Lei H, Poitry-Yamate C, Preitner F, Thorens B, Gruetter R. Neurochemical profile of the mouse hypothalamus using in vivo 1H MRS at 14.1T. NMR Biomed. 2010;23:578–83.
Satoh E, Takahashi A. Experimental diabetes enhances Ca2+ mobilization and glutamate exocytosis in cerebral synaptosomes from mice. J Pharmacol Sci. 2009;109:181. p-p
Huang XT, Li C, Peng XP, Guo J, Yue SJ, Liu W, et al. An excessive increase in glutamate contributes to glucose-toxicity in beta-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci Rep. 2017;7:44120.
Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Biomol Concepts. 2012;3:345–64.
Wang WT, Lee P, Yeh HW, Smirnova IV, Choi IY. Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin-induced diabetes detected using in vivo 1H MR spectroscopy at 9.4 T. J Neurochem. 2012;121:407–17.
Duarte JM, Carvalho RA, Cunha RA, Gruetter R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J Neurochem. 2009;111:368–79.
Lim SI, Song KH, Yoo CH, Woo DC, Choe BY. High-fat diet-induced hyperglutamatergic activation of the hippocampus in mice: a proton magnetic resonance spectroscopy study at 9.4T. Neurochem Int. 2017;114:10–7.
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131.
Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62:2629–34.
Marques EL, Halpern A, Correa Mancini M, de Melo ME, Horie NC, Buchpiguel CA, et al. Changes in neuropsychological tests and brain metabolism after bariatric surgery. J Clin Endocrinol Metab. 2014;99:E2347–52.
Delgado TC, Violante IR, Nieto-Charques L, Cerdan S. Neuroglial metabolic compartmentation underlying leptin deficiency in the obese ob/ob mice as detected by magnetic resonance imaging and spectroscopy methods. J Cerebr Blood F Met. 2011;31:2257–66.
Girault FM, Sonnay S, Gruetter R, Duarte JMN. Alterations of brain energy metabolism in type 2 diabetic Goto-Kakizaki rats measured in vivo by 13C magnetic resonance spectroscopy. Neurotox Res. 2017;1–11.
Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507.
Sonnewald U, Rae C. Pyruvate carboxylation in different model systems studied by C-13 MRS. Neurochem Res. 2010;35:1916–21.
Schonfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013;33:1493–9.
Delgado TC. Glutamate and GABA in appetite regulation. Front Endocrinol. 2013;4:103.
Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;49(Suppl 8):73–5.
Acknowledgements
This research was supported by the Swiss National Science Foundation (grants 148250 to JMND, and 149983 to RG), and by Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenaards and Jeantet Foundations. JMND acknowledges the generous support from the Knut and Alice Wallenberg foundation. We thank A.F. Soares for helping designing the diet administration protocol and monitoring the animals and H. Lei for her technical support. BL designed the study, performed experiments, and wrote the manuscript. AC and JMND performed experiments, and all authors contributed to interpreting the data and writing the manuscript. BL takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lizarbe, B., Cherix, A., Duarte, J.M.N. et al. High-fat diet consumption alters energy metabolism in the mouse hypothalamus. Int J Obes 43, 1295–1304 (2019). https://doi.org/10.1038/s41366-018-0224-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0224-9
This article is cited by
-
Seipin Deficiency Leads to Energy Dyshomeostasis via Inducing Hypothalamic Neuroinflammation and Aberrant Expression of Neuropeptides
NeuroMolecular Medicine (2024)
-
Hypothalamic GABRA5-positive neurons control obesity via astrocytic GABA
Nature Metabolism (2023)
-
Alterations of the glutamatergic system in diabetes mellitus
Metabolic Brain Disease (2023)
-
MAFLD progression contributes to altered thalamus metabolism and brain structure
Scientific Reports (2022)
-
Hepatic microRNA modulation might be an early event to non-alcoholic fatty liver disease development driven by high-fat diet in male mice
Molecular Biology Reports (2022)