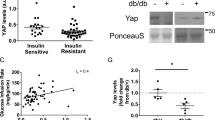
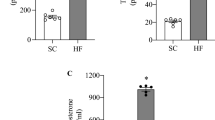
Abstract
Background/objective
The partitioning of glucose toward glycolytic end products rather than glucose oxidation and glycogen storage is evident in skeletal muscle with severe obesity and type 2 diabetes. The purpose of the present study was to determine the possible mechanism by which severe obesity alters insulin-mediated glucose partitioning in human skeletal muscle.
Subjects/methods
Primary human skeletal muscle cells (HSkMC) were isolated from lean (BMI = 23.6 ± 2.6 kg/m2, n = 9) and severely obese (BMI = 48.8 ± 1.9 kg/m2, n = 8) female subjects. Glucose oxidation, glycogen synthesis, non-oxidized glycolysis, pyruvate oxidation, and targeted TCA cycle metabolomics were examined in differentiated myotubes under basal and insulin-stimulated conditions.
Results
Myotubes derived from severely obese subjects exhibited attenuated response of glycogen synthesis (20.3%; 95% CI [4.7, 28.8]; P = 0.017) and glucose oxidation (5.6%; 95% CI [0.3, 8.6]; P = 0.046) with a concomitant greater increase (23.8%; 95% CI [5.7, 47.8]; P = 0.004) in non-oxidized glycolytic end products with insulin stimulation in comparison to the lean group (34.2% [24.9, 45.1]; 13.1% [8.6, 16.4], and 2.9% [−4.1, 12.2], respectively). These obesity-related alterations in glucose partitioning appeared to be linked with reduced TCA cycle flux, as 2-[14C]-pyruvate oxidation (358.4 pmol/mg protein/min [303.7, 432.9] vs. lean 439.2 pmol/mg protein/min [393.6, 463.1]; P = 0.013) along with several TCA cycle intermediates, were suppressed in the skeletal muscle of severely obese individuals.
Conclusions
These data suggest that with severe obesity the partitioning of glucose toward anaerobic glycolysis in response to insulin is a resilient characteristic of human skeletal muscle. This altered glucose partitioning appeared to be due, at least in part, to a reduction in TCA cycle flux.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vinciguerra F, Baratta R, Farina MG, Tita P, Padova G, Vigneri R, et al. Very severely obese patients have a high prevalence of type 2 diabetes mellitus and cardiovascular disease. Acta Diabetol. 2013;50:443–9.
Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes. 2013;37:889–91.
Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health. 2007;121:492–6.
Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31:957–63.
Dohm GL, Tapscott EB, Pories WJ, Dabbs DJ, Flickinger EG, Meelheim D, et al. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988;82:486–94.
Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279.
Kelley DE, Reilly JP, Veneman T, Mandarino LJ. Effects of insulin on skeletal muscle glucose storage, oxidation, and glycolysis in humans. Am J Physiol. 1990;258(6 Pt 1):E923–9.
Friedman JE, Caro JF, Pories WJ, Azevedo JL Jr., Dohm GL. Glucose metabolism in incubated human muscle: effect of obesity and non-insulin-dependent diabetes mellitus. Metabolism. 1994;43:1047–54.
Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–8.
Consoli A, Nurjhan N, Reilly JJ Jr., Bier DM, Gerich JE. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990;8:2038–45.
Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4:R1–R15.
Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–14.
Boyle KE, Zheng D, Anderson EJ, Neufer PD, Houmard JA. Mitochondrial lipid oxidation is impaired in cultured myotubes from obese humans. Int J Obes. 2012;36:1025–31.
Fisher-Wellman KH, Weber TM, Cathey BL, Brophy PM, Gilliam LA, Kane CL, et al. Mitochondrial respiratory capacity and content are normal in young insulin-resistant obese humans. Diabetes. 2014;63:132–41.
Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, et al. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes. 2002;51:901–9.
Hinkley JM, Zou K, Park S, Turner K, Zheng D, Houmard JA. Roux-en-Y gastric bypass surgery enhances contraction-mediated glucose metabolism in primary human myotubes. Am J Physiol Endocrinol Metab. 2017;313:E195–202.
Hinkley JM, Zou K, Park S, Zheng D, Dohm GL, Houmard JA. Differential acute and chronic responses in insulin action in cultured myotubes following from nondiabetic severely obese humans following gastric bypass surgery. Surg Obes Relat Dis. 2017;13:1853–62.
Koek MM, Muilwijk B, van der Werf MJ, Hankemeier T. Microbial metabolomics with gas chromatography/mass spectrometry. Anal Chem. 2006;78:1272–81.
Gaster M, Beck-Nielsen H. The reduced insulin-mediated glucose oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin--evidence from cultured myotubes. Biochim Biophys Acta. 2004;1690:85–91.
Kelleher JK, Bryan BM 3rd, Mallet RT, Holleran AL, Murphy AN, Fiskum G. Analysis of tricarboxylic acid-cycle metabolism of hepatoma cells by comparison of 14CO2 ratios. Biochem J. 1987;246:633–9.
Qvisth V, Hagstrom-Toft E, Moberg E, Sjoberg S, Bolinder J. Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am J Physiol Endocrinol Metab. 2007;292:E709–14.
Berhane F, Fite A, Daboul N, Al-Janabi W, Msallaty Z, Caruso M, et al. Plasma lactate levels increase during hyperinsulinemic euglycemic clamp and oral glucose tolerance test. J Diabetes Res. 2015;2015:102054.
Gaster M. Reduced TCA flux in diabetic myotubes: a governing influence on the diabetic phenotype? Biochem Biophys Res Commun. 2009;387:651–5.
Mandarino LJ, Wright KS, Verity LS, Nichols J, Bell JM, Kolterman OG, et al. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987;80:655–63.
Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab. 2014;11:10.
Rahimi Y, Camporez JP, Petersen MC, Pesta D, Perry RJ, Jurczak MJ, et al. Genetic activation of pyruvate dehydrogenase alters oxidative substrate selection to induce skeletal muscle insulin resistance. Proc Natl Acad Sci USA. 2014;111:16508–13.
Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–44.
Ortenblad N, Mogensen M, Petersen I, Hojlund K, Levin K, Sahlin K, et al. Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta. 2005;1741:206–14.
Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab. 2003;284:E741–7.
Baker PR 2nd, Boyle KE, Koves TR, Ilkayeva OR, Muoio DM, Houmard JA, et al. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity. 2015;23:981–8.
Maples JM, Brault JJ, Shewchuk BM, Witczak CA, Zou K, Rowland N, et al. Lipid exposure elicits differential responses in gene expression and DNA methylation in primary human skeletal muscle cells from severely obese women. Physiol Genomics. 2015;47:139–46.
Maples JM, Brault JJ, Witczak CA, Park S, Hubal MJ, Weber TM, et al. Differential epigenetic and transcriptional response of the skeletal muscle carnitine palmitoyltransferase 1B (CPT1B) gene to lipid exposure with obesity. Am J Physiol Endocrinol Metab. 2015;309:E345–56.
Gaster M. Reduced TCA flux in diabetic myotubes: determined by single defects? Biochem Res Int. 2012;2012:716056.
Gaster M, Nehlin JO, Minet AD. Impaired TCA cycle flux in mitochondria in skeletal muscle from type 2 diabetic subjects: marker or maker of the diabetic phenotype? Arch Physiol Biochem. 2012;118:156–89.
Houmard JA, Pories WJ, Dohm GL. Severe obesity: evidence for a deranged metabolic program in skeletal muscle? Exerc Sport Sci Rev. 2012;40:204–10.
Consitt LA, Bell JA, Koves TR, Muoio DM, Hulver MW, Haynie KR, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes. 2010;59:1407–15.
Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab. 2004;287:E1076–81.
Bikman BT, Zheng D, Pories WJ, Chapman W, Pender JR, Bowden RC, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93:4656–63.
Bjornholm M, Kawano Y, Lehtihet M, Zierath JR. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes. 1997;46:524–7.
Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver MW, et al. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab. 2010;95:3400–10.
Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL. Lipid-induced insulin resistance is prevented in lean and obese myotubes by AICAR treatment. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1692–9.
Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–61.
Acknowledgements
We thank the research volunteers for their dedication and effort to this study. We also thank Gabe Dubis and Angela Clark for assisting in specimen collection. This study was supported by grants from Janssen Research & Development, LLC, Golden LEAF Foundation, National Institutes of Health (DK56112, JAH) and American Heart Association Postdoctoral Fellowship (15POST25080003, KZ). This publication was made possible by Mayo Clinic Metabolomics Resource Core through grant number U24DK100469 from the National Institute of Diabetes and Digestive and Kidney Diseases and originates from the National Institutes of Health Director’s Common Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P.J.H. and J.L. are employed by Johnson & Johnson. G.L.D. and W.J.P. received research grants from Janssen Research & Development, LLC.
Rights and permissions
About this article
Cite this article
Zou, K., Hinkley, J.M., Park, S. et al. Altered tricarboxylic acid cycle flux in primary myotubes from severely obese humans. Int J Obes 43, 895–905 (2019). https://doi.org/10.1038/s41366-018-0137-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0137-7
This article is cited by
-
Undaria pinnatifida improves obesity-related outcomes in association with gut microbiota and metabolomics modulation in high-fat diet-fed mice
Applied Microbiology and Biotechnology (2020)