Abstract
Introduction:
Alkaline phosphatase is implicated in intestinal lipid transport and in the development of obesity. Placental alkaline phosphatase is localised to the microvillous plasma membrane of the placental syncytiotrophoblast at the maternal–fetal interface, but its role is unclear. We investigated the relations of placental alkaline phosphatase activity and mRNA expression with maternal body composition and offspring fat mass in humans.
Methods:
Term human placentas from the UK Birthright cohort (n = 52) and the Southampton Women’s Survey (SWS) (n = 95) were studied. In the Birthright cohort, alkaline phosphatase activity was measured in placental microvillous plasma membrane vesicles. In the SWS, alkaline phosphatase mRNA was measured using Nanostring. Alkaline phosphatase gene expression was compared to other lipid-related genes.
Results:
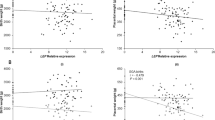
In Birthright samples placental microvillous plasma membrane alkaline phosphatase activity was positively associated with maternal triceps skinfold thickness and BMI (β = 0.04 (95% CI: 0.01–0.06) and β = 0.02 (0.00–0.03) µmol/mg protein/min per SD, P = 0.002 and P = 0.05, respectively) after adjusting for potential confounders. In SWS samples placental alkaline phosphatase mRNA expression in term placenta was positively associated with maternal triceps skinfold (β = 0.24 (0.04, 0.44) SD/SD, P = 0.02), had no association with neonatal %fat mass (β = 0.01 (−0.20 to 0.21) SD/SD, P = 0.93) and was negatively correlated with %fat mass at ages 4 (β = −0.28 (−0.52 to −0.04) SD/SD, P = 0.02), 6–7 (β = −0.25 (−0.49 to −0.02) SD/SD, P = 0.03) years. When compared with placental expression of other genes, alkaline phosphatase expression was positively related to genes including the lysophosphatidylcholine transporter MFSD2A (major facilitator superfamily domain containing 2A, P < 0.001) and negatively related to genes including the fatty acid transport proteins 2 and 3 (P = 0.001, P < 0.001).
Conclusions:
Our findings suggest relationships between placental alkaline phosphatase and both maternal and childhood adiposity. The inverse relationship between placental alkaline phosphatase gene expression and childhood %fat mass suggests that placental alkaline phosphatase may help to protect the foetus from the adverse effects of maternal obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64.
Lewis RM, Desoye G, Placental lipid and fatty acid transfer in maternal overnutrition. Ann Nutr Metab. 2017;70:228–31.
Day PE, Ntani G, Crozier SR, Mahon PA, Inskip HM, Cooper C, et al. Maternal factors are associated with the expression of placental genes involved in amino acid metabolism and transport. PLoS One. 2015;10:e0143653
O’Tierney PF, Lewis RM, McWeeney SK, Hanson MA, Inskip HM, Morgan TK, et al. Immune response gene profiles in the term placenta depend upon maternal muscle mass. Reprod Sci. 2012;19:1041–56.
Lewis RM, Greenwood SL, Cleal JK, Crozier SR, Verrall L, Inskip HM, et al. Maternal muscle mass may influence system A activity in human placenta. Placenta. 2010;31:418–22.
Hirschmugl B, Desoye G, Catalano P, Klymiuk I, Scharnagl H, Payr S, et al. Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int J Obes (Lond). 2017;41:317–23.
Lewis RM, Cleal JK, Hanson MA. Review: placenta, evolution and lifelong health. Placenta. 2012;33:S28–32.
Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem. 2014;29:269–78.
Bashiri A, Katz O, Maor E, Sheiner E, Pack I, Mazor M, et al. Positive placental staining for alkaline phosphatase corresponding with extreme elevation of serum alkaline phosphatase during pregnancy. Arch Gynecol Obstet. 2007;275:211–4.
Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One. 2013;8:e56754.
Desoye G, Hofmann HH, Weiss PA. Insulin binding to trophoblast plasma membranes and placental glycogen content in well-controlled gestational diabetic women treated with diet or insulin, in well-controlled overt diabetic patients and in healthy control subjects. Diabetologia. 1992;35:45–55.
Narisawa S, Huang L, Iwasaki A, Hasegawa H, Alpers DH, Millan JL, et al. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol. 2003;23:7525–30.
Gul SS, Hamilton AR, Munoz AR, Phupitakphol T, Liu W, Hyoju SK, et al. Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice. Appl Physiol Nutr Metab. 2017;42:77–83.
Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci USA. 2013;110:7003–8.
Hernandez-Mosqueira C, Velez-delValle C, Kuri-Harcuch W. Tissue alkaline phosphatase is involved in lipid metabolism and gene expression and secretion of adipokines in adipocytes. Biochim Biophys Acta. 2015;1850:2485–96.
Kusinski LC, Jones CJ, Baker PN, Sibley CP, Glazier JD. Isolation of plasma membrane vesicles from mouse placenta at term and measurement of system A and system beta amino acid transporter activity. Placenta. 2010;31:53–9.
Glazier JD, Jones CJ, Sibley CP. Preparation of plasma membrane vesicles from the rat placenta at term and measurement of Na+ uptake. Placenta. 1990;11:451–63.
Lencel P, Delplace S, Hardouin P, Magne D. TNF-alpha stimulates alkaline phosphatase and mineralization through PPARgamma inhibition in human osteoblasts. Bone. 2011;48:242–9.
Albert JL, Sundstrom SA, Lyttle CR. Estrogen regulation of placental alkaline phosphatase gene expression in a human endometrial adenocarcinoma cell line. Cancer Res. 1990;50:3306–10.
Deng G, Liu G, Hu L, Gum JR Jr, Kim YS. Transcriptional regulation of the human placental-like alkaline phosphatase gene and mechanisms involved in its induction by sodium butyrate. Cancer Res. 1992;52:3378–83.
Chou JY, Takahashi S. Control of placental alkaline phosphatase gene expression in HeLa cells: induction of synthesis by prednisolone and sodium butyrate. Biochemistry. 1987;26:3596–602.
Lalles JP, Orozco-Solis R, Bolanos-Jimenez F, de Coppet P, Le Drean G, Segain JP, et al. Perinatal undernutrition alters intestinal alkaline phosphatase and its main transcription factors KLF4 and Cdx1 in adult offspring fed a high-fat diet. J Nutr Biochem. 2012;23:1490–7.
Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG, et al. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58:443–54.
Calabuig-Navarro V, Haghiac M, Minium J, Glazebrook P, Ranasinghe GC, Hoppel C, et al. Effect of maternal obesity on placental lipid metabolism. Endocrinology. 2017;158:2543–55.
Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16:1694–703.
Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C, et al. Cohort profile: the Southampton Women’s Survey. Int J Epidemiol. 2006;35:42–8.
Godfrey KM, Matthews N, Glazier J, Jackson A, Wilman C, Sibley CP, et al. Neutral amino acid uptake by the microvillous plasma membrane of the human placenta is inversely related to fetal size at birth in normal pregnancy. J Clin Endocrinol Metab. 1998;83:3320–6.
Glazier JD, Jones CJ, Sibley CP. Purification and Na+ uptake by human placental microvillus membrane vesicles prepared by three different methods. Biochim Biophys Acta. 1988;945:127–34.
Crozier SR, Inskip HM, Barker ME, Lawrence WT, Cooper C, Robinson SM, et al. Development of a 20-item food frequency questionnaire to assess a ‘prudent’ dietary pattern among young women in Southampton. Eur J Clin Nutr. 2010;64:99–104.
Leary SD, Godfrey KM, Greenaway LJ, Davill VA, Fall CH. Contribution of the umbilical cord and membranes to untrimmed placental weight. Placenta. 2003;24:276–8.
Armitage P, Berry G . Statistical Methods in Medical Research. 3rd ed. Oxford, UK: Blackwell Science Ltd.; 2002
Rasmussen KM, Yaktine AL (editors). Washington, DC: Weight gain during pregnancy: reexamining the guidelines; National Academies Press, U, S2009.
Solomon AL, Siddals KW, Baker PN, Gibson JM, Aplin JD, Westwood M, et al. Placental alkaline phosphatase de-phosphorylates insulin-like growth factor (IGF)-binding protein-1. Placenta. 2014;35:520–2.
Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. 2015;101:368–75.
Prieto-Sanchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr. 2017;36:513–21.
Madore C, Nadjar A, Delpech JC, Sere A, Aubert A, Portal C, et al. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav Immun. 2014;41:22–31.
de Santis MS, Taricco E, Radaelli T, Spada E, Rigano S, Ferrazzi E, et al. Growth of fetal lean mass and fetal fat mass in gestational diabetes. Ultrasound Obstet Gynecol. 2010;36:328–37.
Acknowledgements
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007–2013), project EarlyNutrition under Grant agreement no. 289346. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (as an NIHR Senior Investigator [NF-SI-0515-10042] and through the NIHR Southampton Biomedical Research Centre) and the European Union’s Seventh Framework Programme (FP7/2007–2013), project ODIN under Grant agreement no. 613977.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hirschmugl, B., Crozier, S., Matthews, N. et al. Relation of placental alkaline phosphatase expression in human term placenta with maternal and offspring fat mass. Int J Obes 42, 1202–1210 (2018). https://doi.org/10.1038/s41366-018-0136-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0136-8