Abstract
Background/objectives
Evidence from animal studies highlights an important role for serotonin (5-HT), derived from gut enterochromaffin (EC) cells, in regulating hepatic glucose production, lipolysis and thermogenesis, and promoting obesity and dysglycemia. Evidence in humans is limited, although elevated plasma 5-HT concentrations are linked to obesity.
Subjects/methods
We assessed (i) plasma 5-HT concentrations before and during intraduodenal glucose infusion (4 kcal/min for 30 min) in non-diabetic obese (BMI 44 ± 4 kg/m2, N = 14) and control (BMI 24 ± 1 kg/m2, N = 10) subjects, (ii) functional activation of duodenal EC cells (immunodetection of phospho-extracellular related-kinase, pERK) in response to glucose, and in separate subjects, (iii) expression of tryptophan hydroxylase-1 (TPH1) in duodenum and colon (N = 39), and (iv) 5-HT content in primary EC cells from these regions (N = 85).
Results
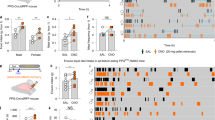
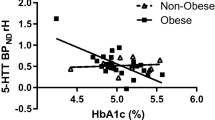
Plasma 5-HT was twofold higher in obese than control responders prior to (P = 0.025), and during (iAUC, P = 0.009), intraduodenal glucose infusion, and related positively to BMI (R2 = 0.334, P = 0.003) and HbA1c (R2 = 0.508, P = 0.009). The density of EC cells in the duodenum was twofold higher at baseline in obese subjects than controls (P = 0.023), with twofold more EC cells activated by glucose infusion in the obese (EC cells co-expressing 5-HT and pERK, P = 0.001), while the 5-HT content of EC cells in duodenum and colon was similar; TPH1 expression was 1.4-fold higher in the duodenum of obese subjects (P = 0.044), and related positively to BMI (R2 = 0.310, P = 0.031).
Conclusions
Human obesity is characterized by an increased capacity to produce and release 5-HT from the proximal small intestine, which is strongly linked to higher body mass, and glycemic control. Gut-derived 5-HT is likely to be an important driver of pathogenesis in human obesity and dysglycemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414.
Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138:659–70.
Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, et al. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol. 2011;301:G519–27.
Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol. 2008;295:G260–72.
Kim M, Cooke HJ, Javed NH, Carey HV, Christofi F, Raybould HE. D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001;121:1400–6.
Symonds EL, Peiris M, Page AJ, Chia B, Dogra H, Masding A, et al. Mechanisms of activation of mouse and human enteroendocrine cells by nutrients. Gut. 2015;64:618–26.
Tanaka T, Mizumoto A, Mochiki E, Haga N, Suzuki H, Itoh Z. Relationship between intraduodenal 5-hydroxytryptamine release and interdigestive contractions in dogs. J Smooth Muscle Res. 2004;40:75–84.
Zelkas L, Raghupathi R, Lumsden AL, Martin AM, Sun E, Spencer NJ, et al. Serotonin-secreting enteroendocrine cells respond via diverse mechanisms to acute and chronic changes in glucose availability. Nutr Metab (Lond). 2015;12:55.
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76.
Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–12.
Watanabe H, Akasaka D, Ogasawara H, Sato K, Miyake M, Saito K, et al. Peripheral serotonin enhances lipid metabolism by accelerating bile acid turnover. Endocrinology. 2010;151:4776–86.
Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16:588–600.
Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015;21:166–72.
Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H, et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun. 2015;6:6794.
Bertrand RL, Senadheera S, Markus I, Liu L, Howitt L, Chen H, et al. A Western diet increases serotonin availability in rat small intestine. Endocrinology. 2011;152:36–47.
Kim HJ, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang JT, et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10:722–31.
Le Beyec J, Pelletier AL, Arapis K, Hourseau M, Cluzeaud F, Descatoire V, et al. Overexpression of gastric leptin precedes adipocyte leptin during high-fat diet and is linked to 5HT-containing enterochromaffin cells. Int J Obes (Lond). 2014;38:1357–64.
Kwak SH, Park BL, Kim H, German MS, Go MJ, Jung HS, et al. Association of variations in TPH1 and HTR2B with gestational weight gain and measures of obesity. Obes (Silver Spring). 2012;20:233–8.
Raghupathi R, Duffield MD, Zelkas L, Meedeniya A, Brookes SJ, Sia TC, et al. Identification of unique release kinetics of serotonin from guinea-pig and human enterochromaffin cells. J Physiol. 2013;591:5959–75.
Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62:3532–41.
Nguyen NQ, Debreceni TL, Bambrick JE, Chia B, Wishart J, Deane AM, et al. Accelerated intestinal glucose absorption in morbidly obese humans: relationship to glucose transporters, incretin hormones, and glycemia. J Clin Endocrinol Metab. 2015;100:968–76.
Vanis L, Gentilcore D, Rayner CK, Wishart JM, Horowitz M, Feinle-Bisset C, et al. Effects of small intestinal glucose load on blood pressure, splanchnic blood flow, glycemia, and GLP-1 release in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1524–31.
Merritt JH, Chamness AF, Allen SJ. Studies on blood-brain barrier permeability after microwave-radiation. Radiat Environ Biophys. 1978;15:367–77.
Blum I, Vered Y, Graff E, Grosskopf Y, Don R, Harsat A, et al. The influence of meal composition on plasma serotonin and norepinephrine concentrations. Metabolism. 1992;41:137–40.
Afarideh M, Behdadnia A, Noshad S, Mirmiranpour H, Mousavizadeh M, Khajeh E, et al. Association of peripheral 5-hydroxyindole-3-acetic acid, a serotonin derivative, with metabolic syndrome and low-grade inflammation. Endocr Pract. 2015;21:711–8.
Takahashi T, Yano M, Minami J, Haraguchi T, Koga N, Higashi K, et al. Sarpogrelate hydrochloride, a serotonin2A receptor antagonist, reduces albuminuria in diabetic patients with early-stage diabetic nephropathy. Diabetes Res Clin Pract. 2002;58:123–9.
Ozeck M, Brust P, Xu H, Servant G. Receptors for bitter, sweet and umami taste couple to inhibitory G protein signaling pathways. Eur J Pharmacol. 2004;489:139–49.
Blackshaw LA, Young RL. Detection and signaling of glucose in the intestinal mucosa-vagal pathway. Neurogastroenterol Motil. 2011;23:591–4.
Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–30.
Ritze Y, Hengelhaupt C, Bardos G, Ernst B, Thurnheer M, D’Haese JG, et al. Altered intestinal neuroendocrine gene expression in humans with. Obes Obes (Silver Spring). 2015;23:2278–85.
Haub S, Ritze Y, Ladel I, Saum K, Hubert A, Spruss A, et al. Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice. J Pharmacol Exp Ther. 2011;339:790–8.
Kim K, Oh CM, Ohara-Imaizumi M, Park S, Namkung J, Yadav VK, et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology. 2015;156:444–52.
Young RL, Lumsden AL, Keating DJ. Gut serotonin is a regulator of obesity and metabolism. Gastroenterology. 2015;149:253–5.
Burke LK, Heisler LK. 5-hydroxytryptamine medications for the treatment of obesity. J Neuroendocrinol. 2015;27:389–98.
Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–72.
Freeman SL, Bohan D, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol. 2006;291:G439–45.
Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–70.
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20.
Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–505.
Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34–43.
Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling-the gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14:412–20.
Acknowledgements
We wish to thank staff of the Gastrointestinal Investigation Unit, Royal Adelaide Hospital and Discipline of Surgery, Flinders Medical Center, for their assistance with the study.
Author contributions
DJK and RLY conceived, designed, supervised the study, obtained study support, acquired data, undertook statistical analyses, and wrote the manuscript; they are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. APL and VMJ obtained study support. CKR, NQN, SST, TW, DF, PR, PH, LS, SLD, DAW acquired human duodenal and colon tissues and performed research endoscopy. ALL assisted in study design, acquired data, undertook statistical analyses, and wrote the manuscript. AMM, GS, NP, NJI, NC, and EWLS advised on study design, acquired, and undertook statistical analyses of data. All authors had access to the study data and reviewed and approved the final manuscript.
Funding
Australian Research Council (grant no. LP150100419) and Pfizer Inc. (NY, USA). Pfizer Inc. was not involved in the study design, sample collection, or analysis of data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Young, R.L., Lumsden, A.L., Martin, A.M. et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes 42, 1880–1889 (2018). https://doi.org/10.1038/s41366-018-0047-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0047-8
This article is cited by
-
Contribution of the microbiome for better phenotyping of people living with obesity
Reviews in Endocrine and Metabolic Disorders (2023)
-
The serotonin transporter sustains human brown adipose tissue thermogenesis
Nature Metabolism (2023)
-
Metabolomics in Bariatric Surgery: Towards Identification of Mechanisms and Biomarkers of Metabolic Outcomes
Obesity Surgery (2021)
-
Clostridium ramosum regulates enterochromaffin cell development and serotonin release
Scientific Reports (2019)
-
The gut microbiota to the brain axis in the metabolic control
Reviews in Endocrine and Metabolic Disorders (2019)