Abstract
Exosomes are vesicles encompassed by a lipid bilayer that are released by various living cells. Exosomal proteins are encapsulated within the membrane or embedded on the surface. As an important type of exosome cargo, exosomal proteins can reflect the physiological status of the parent cell and play an essential role in cell–cell communication. Exosomal proteins can regulate tumor development, including tumor-related immune regulation, microenvironment reconstruction, angiogenesis, epithelial–mesenchymal transition, metastasis, etc. The features of exosomal proteins can provide insight into exosome generation, targeting, and biological function and are potential sources of markers for cancer diagnosis, prognosis, and treatment. Here, we summarize the effects of exosomal proteins on cancer biology, the latest progress in the application of exosomal proteins in cancer diagnosis and prognosis, and the potential contribution of exosomal proteins in cancer therapeutics and vaccines.
Similar content being viewed by others
Introduction
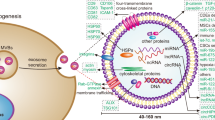
Exosomes arise from multivesicular bodies (MVBs) and are cup-shaped under an electron microscope, with a diameter ranging from 50 to 150 nm. Exosomes are naturally secreted by all kinds of cells and are commonly detected in bodily fluids, including blood, urine, ascites, and saliva1. Exosome cargo includes nucleic acids, proteins, lipids, enzymes, and metabolites that indicate the physiological condition of the parent cell2. Exosomes have been suggested to play a role in cell–cell communication by direct fusion with the cell membrane and through endocytic pathways and ligand–receptor interactions3.
Tumor-derived (TD) exosomes are released by tumor cells and carry substances that can reflect the features of the parental tumor cell4. Therefore, exosomes can be applied as tumor diagnostic markers. As an important form of communication between tumor cells and nontumor cells within the microenvironment, exosomes can play an important role in different stages of tumor development, including tumor-related immune regulation, microenvironment reconstruction, angiogenesis, invasion, and distant metastasis3,5. The expression profile of exosomal proteins is often significantly different in different types and stages of cancer, indicating that these proteins are closely associated with cancer development and progression6. Moreover, other functions of TD exosomes cannot be ignored, including their involvement in restricting immune regulation and enhancing chemoresistance by eliminating chemotherapeutic drugs7,8,9, which might promote primary tumor growth and metastasis.
By examining special subpopulations of exosomes, the cell of origin can be determined based on the increased specificity of exosome cargo. The miRNA content in exosomes is the highest among all kinds of RNAs, and miRNA is stable because it is not easily degraded by RNA enzymes10. miRNA has become a preferred molecule in the study of exosomes due to easy enrichment and sampling11. The mRNAs in exosomes carry abundant genetic information on tumor cells, so the detection of a specific mRNA in exosomes can be used not only to diagnose cancer and evaluate tumor progression but also to monitor the treatment response12. Currently, studies on lncRNAs as tumor biomarkers or prognostic indicators are still in the initial stage, but exosomal lncRNAs are easy to extract and stable in the environment, favorable characteristics for further research13. Compared with other exosome cargo, exosomal proteins have the following advantages in cancer diagnostics: (1) exosomal proteins are stable in exosomes and have a long half-life14. Moreover, exosomal proteins can act directly on target cells, whereas nucleic acids must be transcribed or translated before exerting activity. Therefore, quantitative and qualitative data on exosomal proteins can provide more accurate information than data on other cargo. (2) Compared with other exosome cargo, exosomal surface proteins can be detected in a small sample volume after a relatively simple isolation procedure. Exosomal proteins can provide abundant, stable, sensitive15,16,17,18,19,20,21,22,23, and unique information24,25,26,27 (Table 1). (3) Due to advances in mass spectrometry-based detection technology and the continuous optimization of data collection and analysis methods, the protein coverage and sensitivity of comparative proteomics have improved, and specific exosomal proteins in urine from patients with cancer can be detected14. The application of proteomics tools to analyze posttranslational modifications in exosome research has also increased the depth of exosome proteomics data in the context of cancer pathogenesis, function, and disease associations28. In recent years, researchers have paid increasing attention to exosomal proteins, and numerous studies on exosomal proteins have been recently published. However, few reviews have focused on the function of exosomal proteins in the development, diagnosis, progression, and treatment of cancer29. In this review, we summarize the effects of exosomal proteins on tumor development, highlight the diagnostic and prognostic roles of exosomal proteins in various cancers, and illustrate the potential application of exosomal proteins in cancer therapy. The aim of this review is to provide a comprehensive understanding of the biological mechanisms and clinical value of exosomal proteins, deepen understanding of the roles of exosomal proteins in tumorigenesis and cancer progression, and provide suggestions for further application and investigation.
Proteins on the surface of exosomes
Exosomal proteins are either encapsulated within the lumen or embedded on the surface of exosomes, which enables the subtyping of exosomes based on surface biomarkers without destroying their structure30. Specifically, some proteins (e.g., CD63, TSG101, and Alix) have been recognized as biomarkers for exosomes derived from various cells, while other proteins (e.g., Calnexin) function as negative markers for exosome identification31. Exosomes secreted by cells undergoing pathological processes present distinct compositions that serve as markers of their states. Some proteins (e.g., EGFR, EphA2, and EpCAM) on the surface of exosomes are increasingly used to distinguish TD exosomes from nontumor-derived exosomes6. Metastatic ovarian cancer (OC) cells can release numerous exosomes that carry E-cadherin, an inducer of angiogenesis32. The expression levels of exosomal lipopolysaccharide-binding proteins and E-cadherin were utilized to identify non-small cell lung cancer (NSCLC) and OC cells with metastatic phenotypes33. Metastatic melanoma cells secrete exosomes that carry programmed death ligand-1 (PD-L1), which can bind programmed death-1 (PD-1) on T cells to drive immune checkpoint responses34. In patients with head and neck squamous cell carcinoma, the levels of exosomal PD-L1 are correlated with disease progression, UICC stage, and lymph node invasion (P = 0.0008)35. Another study showed that the detection of PD-L1-positive exosomes in blood samples from patients with pancreatic ductal adenocarcinoma was associated with worse survival36. Pretreating glioblastoma-derived exosomes with anti-PD-1 antibodies reversed the suppression of T cells and prevented cancer progression37. Chen et al.34 demonstrated that PD-L1 on the surface of metastatic melanoma-derived exosomes can suppress CD8+ T cells and promote tumor proliferation, and these processes were affected by anti-PD-1 antibody treatment. Exosomal surface proteins can provide sufficient diagnostic and prognostic information for cancers and can be used to monitor treatment responses. Moreover, exosomal surface proteins may provide insight into the mechanisms of exosome biogenesis38,39,40,41,42,43, targeting44,45, and interactions46,47,48,49. We have summarized the main types of exosomal surface proteins and their functions in Table 2.
The effect of exosomal protein cargo on cancer biology
Exosomal proteins and angiogenesis
Angiogenesis is mediated by vascular endothelial growth factor (VEGF), a proangiogenic factor released by endothelial and tumor cells50. Exosomes carrying VEGF may be crucial for early tumor angiogenesis51. Feng et al.52 found that exosomes can induce angiogenesis in MDA-MB-231 cells by activating a specific form of VEGF called 90 kDa VEGF (VEGF90K). These researchers also found that Hsp90 localized near exosomal VEGF and decreased the efficiency of bevacizumab53. These data indicate that VEGF-activated Hsp90 can promote tumor cell viability. Another research team reported that pancreatic cancer (PC)-derived exosomes can drive angiogenesis by triggering gene expression in human umbilical vein endothelial cells (HUVECs)54. The high levels of phospho-Akt and phospho-ERK1/2 in HUVECs demonstrated the angiogenic function of PC-derived exosomes. Other exosomal proteins that are recognized to facilitate angiogenesis and are potential targets of antiangiogenic drugs include carbonic anhydrase 955, annexin II53, and WNT456. Taken together, these studies have shown that exosomal proteins correlate with angiogenesis and vascular permeability, thereby promoting cancer progression.
Exosomal proteins and epithelial–mesenchymal transition
In cancer, epithelial–mesenchymal transition (EMT) is a specific process in which epithelial cells transform into mesenchymal cells57. There is a relationship between mesenchymal cell-derived exosomes and EMT in epithelial cells58. Simpson et al.59 evaluated intracellular and extracellular protein alterations during EMT (in secretomes, cell membranes, and exosomes) by using EMT models of MDCK (kidney epithelial) cells and H-Ras-transformed MDCK (21D1) cells. Proteomic analyses of the secretomes of MDCK and 21D1 cells showed the remodeling of extracellular matrix (ECM) proteins, including decreased levels of basic membrane components (e.g., collagen type IV) and proteases (e.g., matrix metalloproteinase 1 (MMP-1))60. Proteomic analyses of 21D1 cell-derived exosomes showed a decrease in some epithelial biomarkers (e.g., E-cadherin and EpCAM) but an increase in mesenchymal biomarkers (e.g., vimentin) and proteinases (e.g., MMP-1)61. Therefore, a thorough recognition of the function of exosomal proteins in EMT is needed. Furthermore, therapeutic windows can be exploited during EMT, potentially via pharmacological methods that target exosome biogenesis and secretion mechanisms.
Exosomal proteins and the tumor microenvironment
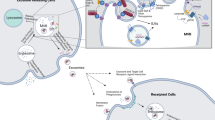
Studies have shown that tumor cells can interact with various types of noncancer cells within the tumor microenvironment (TME)62. The ECM is considered an important part of the TME and serves as a physical and biochemical support for tumor cells to regulate their function63. ECM remodeling can cause quantitative and qualitative alterations in the ECM, which facilitates tumor cell proliferation and metastasis64. As a component of the extracellular environment, tissue-derived exosomes interact with the ECM, and this interaction is an important factor determining the biological effect of these exosomes65. The ability of tissue-derived exosomes to carry tumor promoters and initiate ECM remodeling in the TME is critical for the survival of tumor cells. Exosomal proteins (e.g., extracellular matrix metalloproteinase inducer) have been found to induce ECM remodeling by activating the generation of MMPs in fibroblasts66. MMPs can remodel the ECM by disassembling it and releasing the embedded growth factors, thereby facilitating tumor growth and metastasis. Another study showed that introducing Rab27b into ovarian carcinoma cells can lead to the secretion of exosomes that upregulating MMP2 expression67. In addition to their effects on ECM remodeling, exosomal proteins can also influence the TME by immune regulation. He et al.68 identified a novel function of endoplasmic reticulum (ER) stress-associated exosomes in mediating macrophage cytokine (i.e., IL-10) secretion in the liver cancer microenvironment and indicated the potential to treat liver cancer by targeting an ER stress-exosomal-STAT3 pathway. Hodgkin lymphoma-derived exosomes can facilitate the transformation of normal fibroblasts into tumor-specific fibroblasts, which causes the release of proinflammatory cytokines (e.g., IL-1α, IL-6, and TNF-α), growth factors (e.g., G-CSF and GM-CSF) and proangiogenic factors (e.g., VEGF)28,69. This finding is supported by research on metastatic melanoma-derived exosomes with surface expression of PD-L112. Stimulation with interferon-γ may increase the amount of PD-L1 on the surface of exosomes to suppress the function of CD8+ T cells and promote tumor cell proliferation70. Therefore, in summary, exosomal proteins have variable effects on immune regulation that alter the microenvironment (Fig. 1).
Exosomal proteins and premetastatic behavior
Recently, exosomes have been reported to prime distant organs toward a favorable microenvironment (i.e., premetastatic niche), which promotes the survival and proliferation of tumor cells71. Peinado et al.72 reported that MET was needed for the premetastatic behavior of primary tumors as a result of the exosome-mediated education of bone marrow cells. The expression of Rab proteins, a family involved in exosome generation, was increased in melanoma cells, while silencing RAB27A led to decreased exosome generation, tumor growth, and metastasis73. Costa-Silva et al.74 reported that exosomal macrophage migration inhibitory factor (MIF) induced the secretion of TGF-β from Kupffer cells, which facilitates the biogenesis of fibronectin in hepatic stellate cells. Therefore, fibronectin then traps and confines macrophages and neutrophils in the liver, promoting the construction of premetastatic niches. Hoshino et al.75 found that exosomes released from tumor cells can specifically target recipient cells and prime premetastatic niches in different organs (such as the lung, liver, and brain). In particular, targeting the integrins α6β4 and αvβ5 can reduce exosome interactions and promote lung and liver metastasis, demonstrating that exosomal integrins are involved in predicting organ-specific metastasis. These studies prove that tumor cells can prime target organs by secreting exosomes carrying specific proteins.
Exosomal proteins and drug resistance
Exosome-mediated multidrug resistance is also an important mechanism of cancer development9. Mesenchymal stem cell-derived exosomes have been reported to suppress immune activity and promote chemoresistance in gastric cancer (GC)76,77. The underlying mechanism involves the exosome-mediated activation of calcium-dependent protein kinases and the Raf/MEK/ERK cascade in GC cells78. Drug resistance-related proteins (e.g., LRP and MRP) have been shown to be regulated by mesenchymal stem cell-derived exosomes and to impact the efficacy of 5-fluorouracil and cisplatin79. Tumor cells can also transfer drug efflux pumps to promote drug resistance. P-Glycoprotein80, MDR-181, and ATP-binding cassette subfamily B member 18 are drug efflux pumps that transmit multidrug resistance in prostate cancer, OC, leukemia, and osteosarcoma. The capacity of exosomes to transmit multidrug resistance from an adriamycin-resistant variant of MCF-7 to an adriamycin-sensitive variant of MCF-7 was achieved by controlling P-glycoprotein expression82. The overexpression of P-glycoprotein is regarded as a potential mechanism of drug resistance that affects the movement of anticancer agents and immunosuppressants. Exosomal proteins have been recognized as important mediators of drug resistance, and further studies have demonstrated the application of exosomal proteins to cancer therapy.
The diagnostic value of exosomal proteins in human cancer
Although the contents of exosomes are constantly being redefined, the advantages and uniqueness of exosome cargo have proven their great potential as tumor markers. Proteins located on the surface or within the membrane of exosomes can also be applied as cancer biomarkers. Compared with other types of exosome cargo (DNA, mRNA, miRNA, circRNA, lncRNA, and metabolites), proteins can provide abundant, stable, sensitive, and distinct information. Exosomal proteins were reported as specific diagnostic and prognostic factors for numerous types of cancer, including breast, urinary, lung, gastric, liver, colorectal, ovarian, thyroid, and pancreatic cancers (Fig. 2).
Exosomal proteins obtained from body fluids (plasma, serum, urine, and pleural effusion), tumor tissue, and cultured cell lines have been reported as specific diagnostic and prognostic factors for numerous types of cancer, including breast, urinary, lung, gastric, liver, colorectal, ovarian, thyroid, and pancreatic cancers.
Breast cancer
Worldwide, breast cancer has become the most common cancer among women. Moreover, nearly 50% of breast cancer patients develop metastasis even after receiving systemic therapy, and these patients with metastatic disease have a 5-year survival rate of only approximately 20%83. Research has proven that ADAM10, metalloprotease, CD9, Annexin‐1, and HSP70 are enriched in exosomes isolated from the pleural effusion or serum of breast cancer patients84. Melo et al.85 found that 75% of breast cancer patients had a higher level of surface exosomal GPC1 expression (GPC1+) than the healthy controls. The diagnostic value of fibronectin and developmental endothelial locus‐1 (Del‐1) in breast cancer cell-derived exosomes was suggested by Moon et al.86, with an AUC of 0.961, a sensitivity of 94.70%, and a specificity of 86.36%87. Moreover, the distinct expression pattern of exosomal survivin‐2B in serum is considered a sign of early-stage breast cancer88. When compared with controls who were disease-free for 5 years, these patients showed a dramatic elevation in the serum exosomal survivin-2B level. However, studies are required to illustrate whether exosomal proteins can be used as noninvasive and effective diagnostic markers of breast cancer.
Urinary cancer
Numerous studies have focused on the protein components of bladder and prostate cancer cell-derived exosomes. Wang et al.89 conducted a proteomics study on exosomes derived from HT1376 bladder cancer cells. A total of 353 exosomal proteins were detected by MS, and elevated expression of some exosomal surface proteins, including MUC1, integrin β1, integrin α6, CD36, CD44, CD10, 5T4, basigin, and CD73, was detected. Jeppesen et al.90 reported that the upregulation of several proteins (i.e., vimentin, CK2α, HDGF, annexin 2, and moesin) in bladder cancer cell-derived exosomes is correlated with an increased propensity for metastasis. Urine samples collected after digital rectal examination were reported to be enriched with exosomes released from prostate cells and therefore have a high level of prostate-specific proteins91. Øverbye et al.92 studied urinary exosomal proteins in 16 prostate cancer patients and 15 healthy controls. A total of 246 differentially expressed proteins were detected between the two cohorts, 17 of which showed sensitivities above 60% at 100% specificity, and TM256 had the highest sensitivity (94%). These studies demonstrated the value of urinary exosomes in discovering proteomic biomarkers for the noninvasive diagnosis of urinary cancer.
Lung cancer
Exosomal proteins have shown specific diagnostic value for NSCLC. Wu et al.93 reported that 80% of the exosomes obtained from NSCLC biopsies were EGFR-positive, compared with 2% of those from chronic inflammatory lung tissue. Moreover, exosomes can deliver EGFR to endothelial cells to stimulate the MAPK and Akt pathways, leading to VEGF overexpression and an increase in tumor vascularity. Li et al.94 identified the differentially expressed proteins (including EGFR, GRB2, and SRC) in exosomes from normal bronchial epithelial cells and NSCLC cells. They noted that some proteins related to cell adhesion, ECM, and proteases were highly expressed in NSCLC cell-derived exosomes. Park et al.95 isolated highly purified exosomes from malignant pleural effusion of NSCLC patients and identified potential diagnostic markers, including EGFR, K-Ras, basigin, carcinoembryonic antigen-related cell adhesion molecule 6, claudin1, claudin3, and RAB family proteins. Recently, an EV array was utilized to capture exosomes from the blood of NSCLC patients, and researchers constructed a diagnostic model comprising 30 exosomal proteins96 that had a sensitivity and specificity of approximately 75%. Thus, EV Arrays can be used to not only determine the proteomic profile of exosomes from tumor cells but also potentially diagnose lung cancer97. Sandfeld et al.98 generated an overall survival (OS) prognostic model based on multiple exosomal protein markers and found that NY-ESO-1 was correlated with a worse OS. To conclude, a multiprotein model could classify lung cancer patients with different disease stages and histology types, and exosomal proteins have the potential as diagnostic markers for lung cancer.
Colorectal cancer
Previous studies have identified some protein biomarkers of colorectal cancer (CRC)-derived exosomes, such as CEA, EGFR, MAPK4, PCNA, and keratin 1899. Specifically, there are two types of exosomes (HSA+/DR4 exosomes and HAS-/DR4 exosomes) that carry CD26, CD63, and major histocompatibility complex class molecule II (MHC II)100. These exosomes can contact dendritic cells and strongly potentiate immune responses by decreasing the threshold level of antigen presentation required for activation at the mucosa. Proteomic analyses of CRC cell-derived exosomes have shown the specific expression of many metastatic factors, such as hepatocyte growth factor receptor, S100A8, S100A9, and tenascin C101,102,103. Using 1-D SDS–PAGE and nano-LC–MS/MS analysis, Alin et al.104 identified several exosomal proteins from the ascites of three CRC patients and found that some of these proteins may play roles in tumor development by disrupting epithelial polarity and affecting metastasis, proliferation, immune regulation, and angiogenesis. More recently, a phosphoproteomic analysis of metastatic CRC cell-derived exosomes showed significantly higher levels of phosphorylated proteins than did nonmetastatic CRC cell-derived exosomes, indicating that exosomes might also transfer phosphorylated proteins to target cells105. Thus, more research should be performed to explore the potential application of exosomal proteins in CRC diagnosis and treatment.
Gastric cancer
Some exosomal proteins are involved in the development of GC. TGF-β1, an immunosuppressive cytokine, was detected in exosomes obtained from the serum of GC patients, and TGF-β1 levels were related to the lymphatic metastasis of GC106. Exosomal TGF-β1 can induce the differentiation of regulatory T cells, helping GC cells disrupt normal immune activity in the host. EGFR was found in GC cell-derived exosomes and shown to be delivered to the liver and fuse with the membrane of hepatic stromal cells107. EGFR then suppressed the expression of miR-26a/b and further increased the expression of hepatocyte growth factor in hepatic stromal cells10, promoting the development of a suitable environment for the liver metastasis of GC cells. Yoon et al.108 reported that serum GKN1 levels were significantly higher in healthy controls (median: 6.34 ng/μL, interquartile range (IQR): 5.66–7.54 ng/μL) than in GC patients (median: 3.48 ng/μL, IQR: 2.90–4.11 ng/μL; P < 0.0001). The sensitivity and specificity were 91.2% and 96.0%, respectively, for GC. Furthermore, serum GKN1 levels were shown to distinguish patients with GC from patients with colorectal, liver, lung, breast, pancreatic, ovarian, and prostatic cancer with AUC values greater than 0.94, indicating its value as a GC-specific diagnostic biomarker109. These studies suggest that exosomal proteins are potential diagnostic and prognostic markers for GC.
Liver cancer
Because liver cancer commonly has nonspecific manifestations in the early stage, patients often miss the best opportunity for treatment. Thus, early diagnosis is the most important element for successful liver cancer therapy. In clinical studies, tumor markers (such as α-fetoprotein (AFP)), imaging, and histopathological biopsies are commonly used diagnostic tools for hepatocellular carcinoma (HCC). Approximately 80% of patients with primary liver cancer have elevated levels of AFP in their blood. However, approximately 50% of HCC patients are AFP-negative, so AFP has low sensitivity and specificity for HCC screening. Arbelaiz et al.110 evaluated the protein levels in exosomes from the serum of HCC patients and a healthy cohort and noted that G3BP and PIGR levels were dramatically increased in HCC patients; moreover, the prediction efficacy of these two exosomal proteins for HCC was higher than that of AFP. In contrast to healthy people and patients with cholangiocarcinoma, HCC patients have significantly higher levels of exosomal G3BP. The AUC was 0.904 for HCC versus control, which is higher than that for the widely used marker AFP, and 0.894 for intrahepatic cholangiocarcinoma versus HCC. Therefore, exosomal G3BP could indicate the occurrence of HCC and discriminate HCC from other hepatic diseases111. Fu et al.112 reported that HCC cell-derived exosomes contain SMAD3 protein, which could facilitate the adhesion of HCC cells. The number of SMAD3-positive exosomes was positively associated with HCC stage and pathological grade and was negatively associated with the postsurgical disease-free survival of HCC patients. Wang et al.113 reported that 14-3-3 protein levels were increased in HCC cell-derived exosomes and that 14-3-3 protein could impair antitumor activity through T-cell exhaustion. This group also reported that high 14-3-3 protein levels were related to larger tumor size, poorer tumor differentiation, and more advanced TNM stage. Although several studies have investigated the relationship between exosomal proteins and liver cancer, there remains insufficient in-depth research on the mechanism by which exosomes impact liver cancer formation.
Ovarian cancer
Although serum CA125 has been widely applied as a biomarker for OC, not all OC patients have elevated CA125 levels114. Elevated CA125 is also detected in patients with other malignant and benign diseases. In a study of approximately 70,000 women, the CA125 test and transvaginal ultrasound did not decrease the mortality rate but resulted in unnecessary surgery because of false-positive results115. Therefore, it is necessary to search for novel diagnostic markers for early diagnosis. A study by Runz et al.116 found that CD24 and EpCAM are exosomal cargo of ovarian carcinoma cell lines and malignant ascites. Exosome-mediated proteolytic activity in the TME may promote tumor invasion into the stroma. Claudin 4-positive exosomes were detected in the blood from 32/63 OC patients but only 1/50 healthy controls, suggesting their potential as a highly sensitive and specific indicator of OC117. HSP70, an exosomal surface marker, is highly expressed in OC cell-derived exosomes compared with those derived from healthy controls118. Moreover, exosomal detection using magnetic nanobeads revealed that the serum of OC patients contained a large number of exosomes expressing HER2119. Further analyses are required to characterize and distinguish the populations of exosomes and explore the biological functions of exosomal proteins in vivo.
Thyroid cancer
The diagnosis of thyroid cancer (TC) is challenging. At present, ultrasonography and fine needle aspiration cytology are widely applied for the diagnosis of TC but are not ideal, as indeterminate samples can lead to unnecessary surgeries or missed diagnoses120. Luo D et al.121 found that the levels of some exosomal proteins (SRC, TLN1, ITGB2, and CAPNS1) were associated with EMT in TC patients with lymph node metastasis. Caruso et al.122 reported that HSP protein levels were elevated in TC tissue compared with para-tumor thyroid tissue and benign goiters. The levels of exosomal HSP proteins were dramatically higher in presurgical TC patients than in patients with benign goiters or in TC patients after surgery123. TC patients usually receive thyroidectomy along with radioactive I-131 therapy, followed by thyroid ultrasonography and serum thyroglobulin (Tg) tests124. Huang et al. detected trends in serum Tg and urinary exosomal Tg concentrations in 16 patients with postablative TC125. Serum Tg was not detected in 5 patients after thyroidectomy and radioactive I-131 therapy, while urinary exosomal Tg presented an increasing trend, indicating the possible recurrence of TC. These results suggest that the application of exosomal proteins could greatly contribute to the diagnosis and clinical monitoring of TC.
Pancreatic cancer
Exosomal proteins also have potential application value for PC diagnostics. Buscail et al. observed that GPC1 was enriched in PC cell-derived exosomes126. GPC1-positive exosomes showed great accuracy, with an AUC of 1.0, a sensitivity of 100%, and a specificity of 100%. In contrast, CA 19–9 was inferior at distinguishing PC patients and healthy controls (AUC of 0.739), suggesting that the sensitivity and specificity of exosomal GPC1 in diagnosing PC were higher than those of CA 19–9127. The abundance of GPC1-positive exosomes was correlated with tumor burden and the OS of PC patients128. MIFs were overexpressed in PC cell-derived exosomes, and their suppressive activity can promote the occurrence and metastasis of PC. Compared with patients without PC progression, metastatic PC patients showed a dramatic increase in MIFs, suggesting that exosomal MIFs play essential roles in metastasis and might be predictive markers for liver metastasis. A more recent study reported that the exosomal integrins α6β4 and α6β1 were correlated with the lung metastasis of PC, while integrin αvβ5 was associated with liver metastasis129. Thus, it is important to study the role of exosomal proteins in PC.
The therapeutic value of exosomal proteins in human cancer
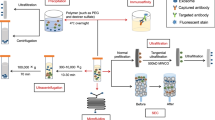
Before the discovery that exosomes are involved in intercellular communication, exosomes were thought to play roles in cancer therapy. It is difficult to explore the impact of exosome-mediate transport on drug delivery because the identification and isolation of specific subpopulations remain challenging130. Therefore, exosomes were further engineered to carry targeting ligands and stimulus-responsive factors. Exosomes can be modified in two different ways, by internal modification (in which the protein cargo within the exosome is modified) and by surface modification (in which the exosomal surface proteins are modified) (Fig. 3). According to timing, the types of modification can be further divided into preisolation and postisolation modification. Preisolation modification is performed before exosomes are isolated from cells131. The parental cells are modified by incubation with the desired therapeutic agents or by gene editing, leading to the encapsulation of therapeutic agents or proteins by the exosomes. In postisolation modification, drugs and therapeutic agents are directly encapsulated into exosomes, which has greater efficiency132. This process involves the coincubation of exosomes and therapeutic agents that can diffuse into the lumen of exosomes along the concentration gradient or the tentative disruption of the exosome membrane to allow the cargo to cross.
Exosomes can be modified by targeting internal proteins (adding protein cargo into the parent cell or exosome) and altering the surface (adding proteins onto the membrane of the parent cell or exosomes). Exosomes containing tumor antigens can stimulate antigen‐presenting cells (APCs) and drive antitumor immune responses in the human body. Engineered exosomes can also directly release antitumor proteins and attack tumor cells.
Engineering exosomes that contain desired proteins to prevent tumor progression is a potential application of exosomes in cancer therapy (Fig. 3)133. A study reported enhanced tumor targeting and antitumor activity by engineered exosomes carrying doxorubicin134. In another study, the parent cells of exosomes were engineered to express Lamp2b fused to αv integrin-specific iRGD peptides, which have sufficient tumor targeting properties in prostate cancer, breast cancer, cervical cancer, and PC models135. A subtype of MVBs called arrestin domain-containing protein 1–mediated microvesicles (ARMMs) was reported to transfer NOTCH receptors to target cells and stimulate downstream gene expression136. Using chimeric proteins that drive the secretion of ARMMs, these microvesicles were shown to deliver the tumor suppressor p53 protein in vivo137. A new hybrid exosome method was reported by Votteler et al.138, who introduced the concept of enveloped protein nanocages (EPNs). By incorporating various engineered proteins, EPNs utilize membrane binding and self-assembly for their biogenesis and deliver their cargo into the cytoplasm of recipient cells139.
The immune functions of exosomes might make them useful as specific drug transportation tools or vaccines for cancer immunotherapy140. Cancer vaccines present tumor antigens to immune cells, thus activating an antitumor immune response141. As TD exosomes carry many tumor antigens, they are involved in antigen presentation and appear to be a possible cancer vaccine142. Since antigen‐presenting cell-derived exosomes are dependent on MHC, they must match the MHC haplotype. However, tumor cell-derived exosomes do not require MHC haplotype matching. Therefore, it is possible to create cell-free anticancer vaccines that do not need to be engineered for every patient. In addition, exosomes carry tumor antigens that are not specific to one type of cancer, so they may be able to protect against a variety of cancers7. Cell hyperthermia is known to result in elevated Hsp70 levels in both cells and exosomes143. Hsp70 can activate dendritic cells and monocytes and stimulate TD exosome‐mediated immune responses144. Moreover, Hsp70 can stimulate natural killer cells to release granzyme B, which induces the apoptosis of tumor cells145. As a result, HSP70 has the potential as an antigen presented on the surface of exosomes to stimulate antitumor responses. Current drugs and treatments for cancer have side effects and long-term complications, and many patients eventually develop multidrug resistance. Therefore, exosomal proteins have the potential to treat various cancers, as indicated by their antitumor effects in numerous in vitro and in vivo studies.
Future perspective and conclusion
Recently, with the popularity of proteomics analyses, the secretion and function of exosomal proteins obtained from cell lines or body fluids have been revealed, and more attention has been given to their role in predicting tumor development. Exosomal surface proteins hold clues to the mechanisms of exosome biogenesis, secretion, protein‒protein interactions, and recipient cell targeting. Exosomal proteins play essential roles in many aspects of cancer, including EMT, ECM remodeling, angiogenesis, tumor-related immune regulation, premetastatic behavior, and therapeutic resistance.
Exosomes from body fluids are selectively enriched with characteristic proteins of cancer lesions. Using known cancer surface marker antibodies fixed on a chip, exosomal surface biomarkers were detected, indicating that the identification of exosomal surface biomarkers can provide biological information on tumors. New biomarkers will be identified, and more sensitive methods will be developed for early cancer diagnosis, prognosis, and therapy evaluation. However, the challenge of exosomal proteomic analyses compared with RNA analyses is the lack of amplification procedures for protein cargo. Moreover, to efficiently integrate the results from different research teams and translate the feasibility of using exosomes in the clinic, we should standardize the methods for exosome isolation and identification.
In summary, looking forward to improvements in technology, exosomal protein content or proteomic profiles may provide not only diagnostic and prognostic clues for cancer patients but also cancer treatment options in the future.
References
Zhang, Y., Liu, Y., Liu, H. & Tang, W. H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 9, 19 (2019).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
Jan, A., Rahman, S., Khan, S., Tasduq, S. & Choi, I. Biology, pathophysiological role, and clinical implications of exosomes: a critical appraisal. Cells 8, 99 (2019).
Gluszko, A. et al. The role of tumor-derived exosomes in tumor angiogenesis and tumor progression. Curr. Issues Pharm. Med. Sci. 32, 193–202 (2019).
da Costa, V. R. et al. Exosomes in the tumor microenvironment: from biology to clinical applications. Cells 10, 2617 (2021).
Hoshino, A. et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 182, 1044–1061.e18 (2020).
Taghikhani, A. et al. Engineered tumor-derived extracellular vesicles: potentials in cancer immunotherapy. Front. Immunol. 11, 275–280 (2020).
Tan, T. et al. Recent advances in understanding the mechanisms of elemene in reversing drug resistance in tumor cells: a review. Molecules 26, 5792 (2021).
Tang, Z., Li, D., Hou, S. & Zhu, X. The cancer exosomes: clinical implications, applications and challenges. Int. J. Cancer 146, 2946–2959 (2020).
Zhang, Y. et al. Screening of non-invasive miRNA biomarker candidates for metastasis of gastric cancer by small RNA sequencing of plasma exosomes. Carcinogenesis 41, 582–590 (2020).
Chen, M. et al. Transcriptome and long noncoding RNA sequencing of three extracellular vesicle subtypes released from the human colon cancer LIM1863 cell line. Sci. Rep. 6, 38397 (2016).
Del Re, M. et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer 118, 820–824 (2018).
Lakshmi, S., Hughes, T. A. & Priya, S. Exosomes and exosomal RNAs in breast cancer: a status update. Eur. J. Cancer 144, 252–268 (2021).
Schey, K. L., Luther, J. M. & Rose, K. L. Proteomics characterization of exosome cargo. Methods 87, 75–82 (2015).
Su, Y. Y. et al. Upregulated expression of serum exosomal miR-375 and miR-1307 enhance the diagnostic power of CA125 for ovarian cancer. J. Ovarian Res. 12, 6 (2019).
Meng, X. et al. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget 7, 16923–16935 (2016).
Jiao, C., Jiao, X., Zhu, A., Ge, J. & Xu, X. Exosomal miR-34s panel as potential novel diagnostic and prognostic biomarker in patients with hepatoblastoma. J. Pediatr. Surg. 52, 618–624 (2017).
Tang, Y. et al. Tumor-derived exosomal miRNA-320d as a biomarker for metastatic colorectal cancer. J. Clin. Lab. Anal. 33, e23004 (2019).
Shi, R. et al. Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget 6, 26971–26981 (2015).
Zhao, R. et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mole. Cancer 17, 68 (2018).
Işin, M. et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front. Genet. 6, 168 (2015).
Liu, X. H. et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol. Cancer 13, 92 (2014).
Zheng, R. et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer 17, 143 (2018).
Pan, B. et al. Identification of serum exosomal hsa-circ-0004771 as a novel diagnostic biomarker of colorectal cancer. Front. Genet. 10, 1096 (2019).
Allenson, K. et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 28, 741–747 (2017).
Castellanos-Rizaldos, E. et al. Exosome-based detection of EGFR T790M in plasma from non–small cell lung cancer patients. Clin. Cancer Res. 24, 2944–2950 (2018).
Möhrmann, L. et al. Liquid biopsies using plasma exosomal nucleic acids and plasma cell-free DNA compared with clinical outcomes of patients with advanced cancers. Clin. Cancer Res. 24, 181–188 (2018).
Repetto, O. et al. Proteomic exploration of plasma exosomes and other small extracellular vesicles in pediatric hodgkin lymphoma: a potential source of biomarkers for relapse occurrence. Diagnostics 11, 917 (2021).
Li, W. et al. Role of exosomal proteins in cancer diagnosis. Mol. Cancer 16, 145 (2017).
Smolarz, M., Pietrowska, M., Matysiak, N., Mielańczyk, Ł. & Widłak, P. Proteome profiling of exosomes purified from a small amount of human serum: the problem of co-purified serum components. Proteomes 7, 18 (2019).
Liang, Y., Lehrich, B. M., Zheng, S. & Lu, M. Emerging methods in biomarker identification for extracellular vesicle‐based liquid biopsy. J. Extracell. Vesicles 10, 1003–1009 (2021).
Tang, M. K. S. et al. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat. Commun. 9, 2270 (2018).
Wang, N. et al. Circulating exosomes contain protein biomarkers of metastatic non‐small‐cell lung cancer. Cancer Sci. 109, 1701–1709 (2018).
Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018).
Theodoraki, M.-N., Yerneni, S. S., Hoffmann, T. K., Gooding, W. E. & Whiteside, T. L. Clinical significance of PD-L1 + exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 24, 896–905 (2018).
Lux, A., Kahlert, C., Grützmann, R. & Pilarsky, C. c-Met and PD-L1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int. J. Mol. Sci. 20, 3305 (2019).
Ricklefs, F. L. et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, 2549–2557 (2018).
Larios, J., Mercier, V., Roux, A. & Gruenberg, J. ALIX- and ESCRT-III–dependent sorting of tetraspanins to exosomes. J. Cell Biol. 219, e201904113 (2020).
Mazurov, D., Barbashova, L. & Filatov, A. Tetraspanin protein CD9 interacts with metalloprotease CD10 and enhances its release via exosomes. FEBS J. 280, 1200–1213 (2013).
Malla, R. R., Pandrangi, S., Kumari, S., Gavara, M. M. & Badana, A. K. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia. Pac. J. Clin. Oncol. 14, 383–391 (2018).
Verweij, F. J. et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol. 217, 1129–1142 (2018).
Blanc, L. & Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 9, 95–106 (2018).
Phuyal, S., Hessvik, N. P., Skotland, T., Sandvig, K. & Llorente, A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 281, 2214–2227 (2014).
Mashouri, L. et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 18, 75 (2019).
Lynch, S. et al. Novel MHC Class I structures on exosomes. J. Immunol. 183, 1884–1891 (2009).
Berenguer, J. et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J. Extracell. Vesicles 7, 1446660 (2018).
Chauhan, S. et al. Surface glycoproteins of exosomes shed by myeloid-derived suppressor cells contribute to function. J. Proteome Res. 16, 238–246 (2017).
Nazari-Shafti, M.-T. et al. A monocyte inspired guiding system: targeting of exosomes to ischemia activated microvascular endothelium. in 48th Annual Meeting German Society for Thoracic, Cardiac, and Vascular Surgery (2019).
Munich, S., Sobo-Vujanovic, A., Buchser, W. J., Beer-Stolz, D. & Vujanovic, N. L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 1, 1074–1083 (2012).
Du, E., Li, X., He, S., Li, X. & He, S. The critical role of the interplays of EphrinB2/EphB4 and VEGF in the induction of angiogenesis. Mol. Biol. Rep. 47, 4681–4690 (2020).
Bhat, A. et al. Exosomes from cervical cancer cells facilitate pro-angiogenic endothelial reconditioning through transfer of Hedgehog–GLI signaling components. Cancer Cell Int. 21, 319 (2021).
Feng, Q. et al. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 8, 14450 (2017).
Maji, S. et al. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol. Cancer Res. 15, 93–105 (2017).
Chiba, M., Kubota, S., Sato, K. & Monzen, S. Exosomes released from pancreatic cancer cells enhance angiogenic activities via dynamin-dependent endocytosis in endothelial cells in vitro. Sci. Rep. 8, 11972 (2018).
Horie, K. et al. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem. Biophys. Res. Commun. 492, 356–361 (2017).
Huang, Z., Yang, M., Li, Y., Yang, F. & Feng, Y. Exosomes derived from hypoxic colorectal cancer cells transfer Wnt4 to normoxic cells to elicit a prometastatic phenotype. Int. J. Biol. Sci. 14, 2094–2102 (2018).
Brabletz, T., Kalluri, R., Nieto, M. A. & Weinberg, R. A. EMT in cancer. Nat. Rev. Cancer 18, 128–134 (2018).
Kim, H. et al. The emerging roles of exosomes as EMT regulators in cancer. Cells 9, 861 (2020).
Greening, D. W. et al. Emerging roles of exosomes during epithelial–mesenchymal transition and cancer progression. Semin. Cell Dev. Biol. 40, 60–71 (2015).
Gopal, S. K., Greening, D. W., Zhu, H.-J., Simpson, R. J. & Mathias, R. A. Transformed MDCK cells secrete elevated MMP1 that generates LAMA5 fragments promoting endothelial cell angiogenesis. Sci. Rep. 6, 28321 (2016).
Shafiq, A. et al. Transglutaminase‐2, RNA‐binding proteins and mitochondrial proteins selectively traffic to MDCK cell‐derived microvesicles following H‐Ras‐induced epithelial–mesenchymal transition. Proteomics 21, 2000221 (2021).
Bu, L. et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene 38, 4887–4901 (2019).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020).
Rilla, K. et al. Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 75–76, 201–219 (2019).
Pickup, M. W., Mouw, J. K. & Weaver, V. M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253 (2014).
Kim, H.-S., Kim, H. J., Lee, M. R. & Han, I. EMMPRIN expression is associated with metastatic progression in osteosarcoma. BMC Cancer 21, 1059 (2021).
Broner, E. C., Onallah, H., Tavor Re’em, T., Davidson, B. & Reich, R. Role of the exosome secretion machinery in ovarian carcinoma: in vitro and in vivo models. J. Oncol. 2020, 1–11 (2020).
Chengqun, H. E. et al. Exosomes derived from endoplasmic reticulum-stressed liver cancer cells enhance the expression of cytokines in macrophages via the STAT3 signaling pathway. Oncol. Lett. 20, 589–600 (2020).
Nagpal, P., Descalzi‐Montoya, D. B. & Lodhi, N. The circuitry of the tumor microenvironment in adult and pediatric Hodgkin lymphoma: cellular composition, cytokine profile, EBV, and exosomes. Cancer Rep. (Hoboken) 4, 109–122 (2021).
Chen, J. et al. PDL1‐positive exosomes suppress antitumor immunity by inducing tumor‐specific CD8+ T cell exhaustion during metastasis. Cancer Sci. 112, 3437–3454 (2021).
Wang, M. et al. Exosomal proteins: key players mediating pre‑metastatic niche formation and clinical implications (Review). Int. J. Oncol. 58, 4 (2021).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Guo, D., Lui, G. Y., Weninger, W., Beaumont, K. A. & Haass, N. K. Rab27a, a novel marker for melanoma metastasis and poor prognosis. Australas. J. Dermatol. 3, 349–360 (2018).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Wang, M. et al. Lymph node metastasis-derived gastric cancer cells educate bone marrow-derived mesenchymal stem cells via YAP signaling activation by exosomal Wnt5a. Oncogene 40, 2296–2308 (2021).
Lee, S. et al. Mesenchymal stem cell-derived exosomes suppress proliferation of T cells by inducing cell cycle arrest through p27kip1/Cdk2 signaling. Immunol. Lett. 225, 16–22 (2020).
Ji, R. et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle 14, 2473–2483 (2015).
Zhou, J. et al. Mesenchymal stem cell derived exosomes in cancer progression, metastasis and drug delivery: a comprehensive review. J. Cancer 9, 3129–3137 (2018).
Pan, Y., Lin, Y. & Mi, C. Cisplatin‐resistant osteosarcoma cell‐derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801‐dependent manner. Cell Biol. Int. 45, 858–868 (2021).
Qi, R. et al. Microfluidic device for the analysis of MDR cancerous cell-derived exosomes’ response to nanotherapy. Biomed. Microdevices 21, 35 (2019).
Li, C., Wang, Y., Xu, N. & Liu, X. Effects of Michigan Cancer Foundation-7/A new adriamycin cell-derived exosomes on MCF-7 cell apoptosis and drug sensitivity through ubiquitin carboxyl-terminal hydrolase L1. J. Biomater. Tissue Eng. 10, 1780–1785 (2020).
Registry, P. C. Global cancer observatory. Malays. Cancer Stat. 9, 706–712 (2019).
Risha, Y., Minic, Z., Ghobadloo, S. M. & Berezovski, M. V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 10, 13572 (2020).
Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 (2015).
Moon, P.-G. et al. Fibronectin on circulating extracellular vesicles as a liquid biopsy to detect breast cancer. Oncotarget 7, 40189–40199 (2016).
Moon, P.-G. et al. Identification of developmental endothelial locus-1 on circulating extracellular vesicles as a novel biomarker for early breast cancer detection. Clin. Cancer Res 22, 1757–1766 (2016).
Li, K., Liu, T., Chen, J., Ni, H. & Li, W. Survivin in breast cancer–derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J. Biol. Chem. 295, 13737–13752 (2020).
Wang, Y.-T. et al. Proteomic analysis of exosomes for discovery of protein biomarkers for prostate and bladder cancer. Cancers (Basel) 12, 2335 (2020).
Jeppesen, D. K. et al. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics 14, 699–712 (2014).
Dhondt, B. et al. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density‐based fractionation of urine. J. Extracell. Vesicles 9, 1736935 (2020).
Øverbye, A. et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget 6, 30357–30376 (2015).
Wu, S. et al. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol. Cancer 20, 17 (2021).
Clark, D. J., Fondrie, W. E., Yang, A. & Mao, L. Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J. Proteom. 133, 161–169 (2016).
Park, J. O. et al. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics 13, 2125–2134 (2013).
Fan, Y. et al. High-sensitive and multiplex biosensing assay of NSCLC-derived exosomes via different recognition sites based on SPRi array. Biosens. Bioelectron. 154, 112066 (2020).
Sandfeld-Paulsen, B. et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 10, 1595–1602 (2016).
Jakobsen, K. R. et al. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles 4, 26659 (2015).
Xiao, Y. et al. Exosomes as potential sources of biomarkers in colorectal cancer. Cancer Lett. 476, 13–22 (2020).
Mallegol, J. et al. T84-intestinal epithelial exosomes bear MHC Class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 132, 1866–1876 (2007).
Suwakulsiri, W. et al. Proteomic profiling reveals key cancer progression modulators in shed microvesicles released from isogenic human primary and metastatic colorectal cancer cell lines. Biochim. Biophys. Acta Proteins Proteom. 1867, 140171 (2019).
Novikova, S. et al. Proteomic approach for searching for universal, tissue-specific, and line-specific markers of extracellular vesicles in lung and colorectal adenocarcinoma cell lines. Int. J. Mol. Sci. 21, 6601 (2020).
Sun, Z. et al. Proteomics-based identification of candidate exosomal glycoprotein biomarkers and their value for diagnosing colorectal cancer. Front. Oncol. 11, 1293–1300 (2021).
Rai, A. et al. Exosomes derived from human primary and metastatic colorectal cancer cells contribute to functional heterogeneity of activated fibroblasts by reprogramming their proteome. Proteomics 19, 1800148 (2019).
Li, C. et al. Integrated omics of metastatic colorectal cancer. Cancer Cell 38, 734–747.e9 (2020).
Im, K. et al. The comparison of exosome and exosomal cytokines between young and old individuals with or without gastric cancer. Int. J. Gerontol. 12, 233–238 (2018).
Zhang, H. et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 8, 15016 (2017).
Yoon, J. H., Park, Y. G., Nam, S. W. & Park, W. S. The diagnostic value of serum gastrokine 1 (GKN1) protein in gastric cancer. Cancer Med. 8, 5507–5514 (2019).
Yoon, J. H. et al. Gastrokine 1 protein is a potential theragnostic target for gastric cancer. Gastric Cancer 21, 956–967 (2018).
Arbelaiz, A. et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 66, 1125–1143 (2017).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391, 1301–1314 (2018).
Fu, Q. et al. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene 37, 6105–6118 (2018).
Wang, X. et al. 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 9, 159 (2018).
Charkhchi, P. et al. CA125 and ovarian cancer: a comprehensive review. Cancers (Basel) 12, 3730 (2020).
Ahmed, A. A. & Abdou, A. M. Diagnostic accuracy of CA125 and HE4 in ovarian carcinoma patients and the effect of confounders on their serum levels. Curr. Probl. Cancer 43, 450–460 (2019).
Runz, S. et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol. Oncol. 107, 563–571 (2007).
Esfandyari, S. et al. Exosomes as biomarkers for female reproductive diseases diagnosis and therapy. Int. J. Mol. Sci. 22, 2165 (2021).
Chanteloup, G. et al. Membrane-bound exosomal HSP70 as a biomarker for detection and monitoring of malignant solid tumours: a pilot study. Pilot Feasibility Stud. 6, 35 (2020).
Sharma, S. et al. Exosomal miRNAs and proteins signature as prognostic biomarkers for early stage epithelial ovarian cancer. J. Extracell. Vesicles 8, 60–61 (2019).
Tan, L., Tan, Y. S. & Tan, S. Diagnostic accuracy and ability to reduce unnecessary FNAC: a comparison between four Thyroid Imaging Reporting Data System (TI-RADS) versions. Clin. Imaging 65, 133–137 (2020).
Luo, D. et al. Proteomics study of serum exosomes from papillary thyroid cancer patients. Endocr. Relat. Cancer 25, 879–891 (2018).
Caruso Bavisotto, C. et al. Exosomal HSP60: a potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev. Mol. Diagn. 17, 815–822 (2017).
Caruso Bavisotto, C. et al. Immunomorphological pattern of molecular chaperones in normal and pathological thyroid tissues and circulating exosomes: potential use in clinics. Int. J. Mol. Sci. 20, 4496 (2019).
Rageh, T. M., Abdou, A. G., Elkhouly, E. A., Abou El- Ela, D. H. & Zidan, M. A. Preoperative significance of thyroglobulin, thyroid stimulating hormone and thyroglobulin antibody in differentiated papillary thyroid carcinoma. Int. Surg. J. 6, 4229 (2019).
Huang, T. & Wang, C. Urinary exosomal thyroglobulin: a new biomarker for post-operative follow-up in patients with thyroid cancer. Thyroid 9, 23–27 (2018).
Buscail, E. et al. High clinical value of liquid biopsy to detect circulating tumor cells and tumor exosomes in pancreatic ductal adenocarcinoma patients eligible for up-front surgery. Cancers (Basel) 11, 1656 (2019).
Zhou, C. et al. High levels of serum glypican-1 indicate poor prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 7, 5525–5533 (2018).
Frampton, A. E. et al. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget 9, 19006–19013 (2018).
Casari, I., Howard, J. A., Robless, E. E. & Falasca, M. Exosomal integrins and their influence on pancreatic cancer progression and metastasis. Cancer Lett. 507, 124–134 (2021).
Si, Y. et al. Targeted exosomes for drug delivery: biomanufacturing, surface tagging, and validation. Biotechnol. J. 15, 1900163 (2020).
Kučuk, N., Primožič, M., Knez, Ž. & Leitgeb, M. Exosomes engineering and their roles as therapy delivery tools, therapeutic targets, and biomarkers. Int. J. Mol. Sci. 22, 9543 (2021).
Villata, S., Canta, M. & Cauda, V. EVs and bioengineering: from cellular products to engineered nanomachines. Int. J. Mol. Sci. 21, 6048 (2020).
Gutierrez‐Millan, C., Calvo Díaz, C., Lanao, J. M. & Colino, C. I. Advances in exosomes‐based drug delivery systems. Macromol. Biosci. 21, 2000269 (2021).
Schindler, C. et al. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS One 14, e0214545 (2019).
Li, Z. et al. Fusion protein engineered exosomes for targeted degradation of specific RNAs in lysosomes: a proof‐of‐concept study. J. Extracell. Vesicles 9, 1816710 (2020).
Wang, Q. & Lu, Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat. Commun. 8, 709 (2017).
Wang, Q. et al. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 9, 960 (2018).
Votteler, J. et al. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature 540, 292–295 (2016).
Ovchinnikova, L. A. et al. Reprogramming extracellular vesicles for protein therapeutics delivery. Pharmaceutics 13, 768 (2021).
Xu, Z., Zeng, S., Gong, Z. & Yan, Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol. Cancer 19, 160 (2020).
Pi, Y.-N., Xia, B.-R., Jin, M.-Z., Jin, W.-L. & Lou, G. Exosomes: powerful weapon for cancer nano-immunoengineering. Biochem. Pharmacol. 186, 114487 (2021).
Arima, Y., Liu, W., Takahashi, Y., Nishikawa, M. & Takakura, Y. Effects of localization of antigen proteins in antigen-loaded exosomes on efficiency of antigen presentation. Mol. Pharm. 16, 2309–2314 (2019).
Chanteloup, G. et al. Monitoring HSP70 exosomes in cancer patients’ follow up: a clinical prospective pilot study. J. Extracell. Vesicles 9, 1766192 (2020).
Albakova, Z., Armeev, G. A., Kanevskiy, L. M., Kovalenko, E. I. & Sapozhnikov, A. M. HSP70 multi-functionality in cancer. Cells 9, 587 (2020).
Taha, E. A., Ono, K. & Eguchi, T. Roles of extracellular HSPs as biomarkers in immune surveillance and immune evasion. Int. J. Mol. Sci. 20, 4588 (2019).
Funding
This study was supported by grants from the National Natural Science Foundation (82173245), Post-Doctoral Research Project, West China Hospital, Sichuan University (2019HXBH043), Sichuan Science and Technology Program of China (2020YFS0208), the 1•3•5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital Sichuan University (2021HXFH005), and the Science and Technology Achievement Transformation Project, West China Hospital, Sichuan University (CGZH21004).
Author information
Authors and Affiliations
Contributions
The first author of this manuscript is X.W. X.W., J.H., and J.L. conducted the literature retrieval and data collection. X.W. and W.C. summarized the literature and wrote the first draft. Z.L. and G.L. were involved in revising the manuscript. J.L., G.L., and Z.L. made substantial contributions to the conception and design of the manuscript. All authors approve the version of this manuscript for publication and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Huang, J., Chen, W. et al. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp Mol Med 54, 1390–1400 (2022). https://doi.org/10.1038/s12276-022-00855-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00855-4
This article is cited by
-
Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance
Molecular Cancer (2024)
-
miR-142-3p encapsulated in T lymphocyte-derived tissue small extracellular vesicles induces Treg function defect and thyrocyte destruction in Hashimoto’s thyroiditis
BMC Medicine (2023)
-
Thermoresponsive M1 macrophage-derived hybrid nanovesicles for improved in vivo tumor targeting
Drug Delivery and Translational Research (2023)
-
The role of exosomes and their enhancement strategies in the treatment of osteoarthritis
Human Cell (2023)