Abstract
Background
It is still not clear that whether the expression levels of matrix metalloproteinases (MMPs) family are associated with cardiovascular and cerebrovascular diseases (CCDs) in genetic level. We explored the causal role of 12 members of MMPs in CCDs with mendelian randomization (MR) method to facilitate further exploring the underlying mechanisms.
Methods
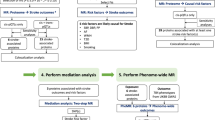
The relationship between MMPs and CCDs including intracerebral hemorrhage (ICH), hypertension, coronary heart disease (CHD), atrial fibrillation (AF), and outstanding risk factors of type II diabetes were determined with the inverse variance-weighted (IVW) method. The sensitivity analyses including MR-Egger regression, weighted median estimation, and MR pleiotropy residual sum and outlier were utilized to test the robustness of the results generated from the MR method.
Results
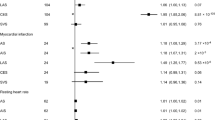
We found that a higher serum level of MMP-12 was related to a lower risk of ICH (OR = 0.8287, 95% CI: 0.7526–0.9125, p = 0.00013), but not hypertension, CHD, type II diabetes or AF. And our study also revealed that a higher serum level of MMP-8 could result in a lower risk of hypertension (OR = 0.9976, 95% CI: 0.9964–0.9988, p = 0.00012) and AF (OR = 0.9851, 95% CI: 0.9741–0.9963, p = 0.0092), but not ICH, CHD or type II diabetes. All other members of MMPs other than MMP-8 and MMP-12 showed no statistical association with CCDs according to this study. Sensitivity analyses confirmed the reliability of our results.
Conclusions
We provided statistical evidences for a potential causal relationship between MMP-12 and ICH, as well as MMP-8 and hypertension, while other MMPs showed weaker association with CCDs. The underlying mechanisms need to be established in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The original contributions presented in the study are in cluded in the article. Further inquiries can be directed to the corresponding author.
References
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the global burden of disease study 2016. Lancet .2017;390:1151–210.
Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–48. https://doi.org/10.1161/CIRCRESAHA.116.308413.
Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet .2017;389:2239–51.
International Diabetes Federation. IDF Diabetes Atlas, 7th edn. 2015. http://www.diabetesatlas.org/ (accessed Oct 6, 2016).
Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36:1481–507.
Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharm. 2018;81:241–330.
Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73.
Lattanzi S, Di Napoli M, Ricci S, Divani AA. Matrix metalloproteinases in acute intracerebral hemorrhage. Neurotherapeutics .2020;17:484–96.
Xiao N, Liu TL, Li H, Xu HC, Ge J, Wen HY, et al. Low serum uric acid levels promote hypertensive intracerebral hemorrhage by disrupting the smooth muscle cell-elastin contractile unit and upregulating the Erk1/2-MMP Axis. Transl Stroke Res. 2020;11:1077–94.
Ezhov M, Safarova M, Afanasieva O, Mitroshkin M, Matchin Y, Pokrovsky S. Matrix metalloproteinase 9 as a predictor of coronary atherosclerotic plaque instability in stable coronary heart disease patients with elevated lipoprotein (a) levels. Biomolecules .2019;9:129.
Lyall DM, Celis-Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, et al. Association of body mass index with cardiometabolic disease in the U.K. biobank: a mendelian randomization study. JAMA Cardiol. 2017;2:882–9.
Smith GD, Ebrahim S. ‘Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22.
Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in U.K. Biobank: a Mendelian randomization study. Eur Heart J. 2020;41:221–6.
Jia Y, Guo D, Zhang K, Yang P, Zang Y, Sun L, et al. Causal associations of serum matrix metalloproteinase-8 level with ischaemic stroke and ischaemic stroke subtypes: a Mendelian randomization study. Eur J Neurol. 2021;28:2543–51.
Cárcel-Márquez J, Cullell N, Muiño E, Gallego-Fabrega C, Lledós M, Ibañez L, et al. Causal Effect of MMP-1 (Matrix Metalloproteinase-1), MMP-8, and MMP-12 levels on ischemic stroke: a Mendelian randomization study. Stroke .2021;52:e316–e320.
Suhre K, Arnold M, Bhagwat AM, Cotton RJ, Engelke R, Raffler J, et al. Connecting genetic risk to disease endpoints through the human blood plasma proteome. Nat Commun. 2017;8:14357.
Woo D, Falcone GJ, Devan WJ, Brown WM, Biffi A, Howard TD, et al. International stroke genetics consortium. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. 2014;94:511–21.
FinnGen-Tutkimushanke Vie Suomalaiset Löytöretkelle Genomitietoon [Internet]. In: FinnGen. Available at: https://www.finngen.fi/fi/finngen_tutkimushanke_vie_suomalaiset_loytoretkelle_genomitietoon.
Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50:1234–9.
Tanha HM, Sathyanarayanan A. International headache genetics consortium, nyholt DR. Genetic overlap and causality between blood metabolites and migraine. Am J Hum Genet. 2021;108:2086–98.
Wu P, Zhang X, Zhou P, Zhang W, Li D, Lv M, et al. Assessment of bidirectional relationships between polycystic ovary syndrome and periodontitis: insights from a Mendelian randomization analysis. Front Genet. 2021;12:644101.
Wang K, Qu M, Ding L, Shi X, Wang C, Cheng S, et al. Liver and kidney function biomarkers, blood cell traits and risk of severe COVID-19: a Mendelian randomization study. Front Genet. 2021;12:647303.
Zou X, Wang L, Xiao L, Xu Z, Yao T, Shen M, et al. Deciphering the irregular risk of stroke increased by obesity classes: a stratified Mendelian randomization study. Front Endocrinol (Lausanne). 2021;12:750999.
Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020;18:312.
Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–42.
Baumeister SE, Karch A, Bahls M, Teumer A, Leitzmann MF, Baurecht H. Physical activity and risk of Alzheimer disease: A 2-sample mendelian randomization study. Neurology .2020;95:e1897–e1905.
Burgess S, Foley CN, Zuber V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu Rev Genom Hum Genet. 2018;19:303–27.
Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2020;4:186.
Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Major depressive disorder working group of the psychiatric genomics consortium. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiatry. 2019;76:399–408.
Bowden J, Davey, Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA 2017;318:1925–6.
Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34:2926–40.
Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics .2016;32:3207–9.
An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10.
García-Cabo C, López-Cancio E. Exercise and stroke. Adv Exp Med Biol. 2020;1228:195–203.
Badimon L, Chagas P, Chiva-Blanch G. Diet and cardiovascular disease: effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr Med Chem. 2019;26:3639–51.
Francula-Zaninovic S, Nola IA. Management of measurable variable cardiovascular disease’ risk factors. Curr Cardiol Rev. 2018;14:153–63.
Yeung CHC, Schooling CM. Systemic inflammatory regulators and risk of Alzheimer’s disease: a bidirectional Mendelian-randomization study. Int J Epidemiol. 2021;50:829–40.
Dieffenbach PB, Mallarino Haeger C, Rehman R, Corcoran AM, Coronata AMF, Vellarikkal SK, et al. A novel protective role for matrix metalloproteinase-8 in the pulmonary vasculature. Am J Respir Crit Care Med. 2021;204:1433–51.
Stouffer GA, Hu Z, Sajid M, Li H, Jin G, Nakada MT, et al. Beta3 integrins are upregulated after vascular injury and modulate thrombospondin- and thrombin-induced proliferation of cultured smooth muscle cells. Circulation 1998;97:907–15.
Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–93.
Fang C, Wen G, Zhang L, Lin L, Moore A, Wu S, et al. An important role of matrix metalloproteinase-8 in angiogenesis in vitro and in vivo. Cardiovasc Res. 2013;99:146–55.
Chong M, Sjaarda J, Pigeyre M, Mohammadi-Shemirani P, Lali R, Shoamanesh A, et al. Novel drug targets for ischemic stroke identified through mendelian randomization analysis of the blood proteome. Circulation .2019;140:819–30.
Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731–42.
Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol. 2007;207:227–37.
Shin JW, Kang HC, Shim J, Sohn NW. Scutellaria baicalensis attenuates blood-brain barrier disruption after intracerebral hemorrhage in rats. Am J Chin Med. 2012;40:85–96.
Johnson JL, Jenkins NP, Huang WC, Di Gregoli K, Sala-Newby GB, Scholtes VP, et al. Relationship of MMP-14 and TIMP-3 expression with macrophage activation and human atherosclerotic plaque vulnerability. Mediat Inflamm. 2014;2014:276457.
Sasaki T, Nakamura K, Sasada K, Okada S, Cheng XW, Suzuki T, et al. Matrix metalloproteinase-2 deficiency impairs aortic atherosclerotic calcification in ApoE-deficient mice. Atherosclerosis. 2013;227:43–50.
Acknowledgements
We thank the Genetic Investigation of UK Biobank, FinnGen Consortium, MRC-IEU database, the MEGASTROKE GWAS dataset, and the International Stroke Genetics Consortium and all concerned investigators for sharing GWAS summary statistics on MMPs and CCDs.
Funding
This study was supported by the National Science & Technology Fundamental Resources Investigation Program of China to LZ (No. 2018FY100900), The Hunan Provincial Natural Science Foundation of China Grant to YZ (No. 2021JJ30923), The Provincial Science and Technology Innovation Leading Talents Project to LZ (No. 2021RC4014), National Clinical Research Center for Geriatric Disorders (XiangYa Hospital).
Author information
Authors and Affiliations
Contributions
LZ and XZ designed the research and determined the structure of the paper. XZ, LX, and LW selected the references and contributed to the writing. XZ, LX, and LW helped to analyze the results of the study. LZ and YZ contributed to the revision and finalization of the article. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Since this study was a retrospective study based on the public GWAS database as the published source, ethics approval was not required. Informed consent was obtained from all individual participants included in the public GWAS study.
Consent for publication
All data in this MR study were obtained from published GWAS studies, and the participants had signed informed consent in the published original GWAS study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, X., Wang, L., Zeng, Y. et al. Illuminating the potential causality of serum level of matrix metalloproteinases and the occurrence of cardiovascular and cerebrovascular diseases: a Mendelian randomization study. J Hum Genet 68, 615–624 (2023). https://doi.org/10.1038/s10038-023-01154-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-023-01154-0