Abstract
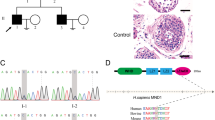
Non-obstructive azoospermia (NOA) is characterized by the failure of sperm production due to testicular disorders and represents the most severe form of male infertility. Growing evidences have indicated that gene defects could be the potential cause of NOA via genome-wide sequencing approaches. Here, bi-allelic deleterious variants in meiosis inhibitor protein 1 (MEI1) were identified by whole-exome sequencing in four Chinese patients with NOA. Testicular pathologic analysis and immunohistochemical staining revealed that spermatogenesis is arrested at spermatocyte stage, with defective programmed DNA double-strand breaks (DSBs) homoeostasis and meiotic chromosome synapsis in patients carrying the variants. In addition, our results showed that one missense variant (c.G186C) reduced the expression of MEI1 and one frameshift variant (c.251delT) led to truncated proteins of MEI1 in in vitro. Furthermore, the missense variant (c.T1585A) was assumed to affect the interaction between MEI1 and its partners via bioinformatic analysis. Collectively, our findings provide direct genetic and functional evidences that bi-allelic variants in MEI1 could cause defective DSBs homoeostasis and meiotic chromosome synapsis, which subsequently lead to meiosis arrest and male infertility. Thus, our study deepens our knowledge of the role of MEI1 in male fertility and provides a novel insight to understand the genetic aetiology of NOA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author, (CY), on reasonable request.
References
Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017;5:544–53.
Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol. 2007;178:608–12.
Yao C, Yang C, Zhao L, Li P, Tian R, Chen H, et al. Bi-allelic SHOC1 loss-of-function mutations cause meiotic arrest and non-obstructive azoospermia. J Med Genet. 2021;58:679–86.
Li P, Ji Z, Zhi E, Zhang Y, Han S, Zhao L, et al. Novel bi-allelic MSH4 variants causes meiotic arrest and non-obstructive azoospermia. Reprod Biol Endocrinol. 2022;20:21.
Kasak L, Laan M. Monogenic causes of non-obstructive azoospermia: challenges, established knowledge, limitations and perspectives. Hum Genet. 2021;140:135–54.
Li Y, Wu Y, Zhou J, Zhang H, Zhang Y, Ma H, et al. A recurrent ZSWIM7 mutation causes male infertility resulting from decreased meiotic recombination. Hum Reprod. 2021;36:1436–45.
Nagirnaja L, Morup N, Nielsen JE, Stakaitis R, Golubickaite I, Oud MS, et al. Variant PNLDC1, defective piRNA processing, and azoospermia. N Engl J Med. 2021;385:707–19.
Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet. 2013;14:794–806.
Baudat F, de Massy B. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res. 2007;15:565–77.
Hunter N. Meiotic Recombination: the essence of heredity. Cold Spring Harb Perspect Biol. 2015;7:a0016618.
Libby BJ, De La Fuente R, O’Brien MJ, Wigglesworth K, Cobb J, Inselman A, et al. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev Biol. 2002;242:174–87.
Sato H, Miyamoto T, Yogev L, Namiki M, Koh E, Hayashi H, et al. Polymorphic alleles of the human MEI1 gene are associated with human azoospermia by meiotic arrest. J Hum Genet. 2006;51:533–40.
Libby BJ, Reinholdt LG, Schimenti JC. Positional cloning and characterization of Mei1, a vertebrate-specific gene required for normal meiotic chromosome synapsis in mice. Proc Natl Acad Sci USA. 2003;100:15706–11.
Reinholdt LG, Schimenti JC. Mei1 is epistatic to Dmc1 during mouse meiosis. Chromosoma. 2005;114:127–34.
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45.
Dong J, Zhang H, Mao X, Zhu J, Li D, Fu J, et al. Novel biallelic mutations in MEI1: expanding the phenotypic spectrum to human embryonic arrest and recurrent implantation failure. Hum Reprod. 2021;36:2371–81.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–89.
Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D44.
Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82.
Shin YH, Choi Y, Erdin SU, Yatsenko SA, Kloc M, Yang F, et al. Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis. PLoS Genet. 2010;6:e1001190.
Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754.
Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–36.
He WB, Tu CF, Liu Q, Meng LL, Yuan SM, Luo AX, et al. DMC1 mutation that causes human non-obstructive azoospermia and premature ovarian insufficiency identified by whole-exome sequencing. J Med Genet. 2018;55:198–204.
Caburet S, Todeschini AL, Petrillo C, Martini E, Farran ND, Legois B, et al. A truncating MEIOB mutation responsible for familial primary ovarian insufficiency abolishes its interaction with its partner SPATA22 and their recruitment to DNA double-strand breaks. EBioMedicine. 2019;42:524–31.
Chen M, Yao C, Qin Y, Cui X, Li P, Ji Z, et al. Mutations of MSH5 in nonobstructive azoospermia (NOA) and rescued via in vivo gene editing. Signal Transduct Target Ther. 2022;7:1.
Yao C, Hou D, Ji Z, Pang D, Li P, Tian R, et al. Bi-allelic SPATA22 variants cause premature ovarian insufficiency and nonobstructive azoospermia due to meiotic arrest. Clin Genet. 2022;101:507–16.
Krausz C, Riera-Escamilla A, Moreno-Mendoza D, Holleman K, Cioppi F, Algaba F, et al. Genetic dissection of spermatogenic arrest through exome analysis: clinical implications for the management of azoospermic men. Genet Med. 2020;22:1956–66.
Ben Khelifa M, Ghieh F, Boudjenah R, Hue C, Fauvert D, Dard R, et al. A MEI1 homozygous missense mutation associated with meiotic arrest in a consanguineous family. Hum Reprod. 2018;33:1034–37.
Dougherty DA. The cation-pi interaction. Acc Chem Res. 2013;46:885–93.
Crowley PB, Golovin A. Cation-pi interactions in protein-protein interfaces. Proteins. 2005;59:231–9.
Young L, Jernigan RL, Covell DG. A role for surface hydrophobicity in protein-protein recognition. Protein Sci. 1994;3:717–29.
Keskin O, Gursoy A, Ma B, Nussinov R. Principles of protein-protein interactions: what are the preferred ways for proteins to interact? Chem Rev. 2008;108:1225–44.
Vrielynck N, Chambon A, Vezon D, Pereira L, Chelysheva L, De Muyt A, et al. A DNA topoisomerase VI-like complex initiates meiotic recombination. Science. 2016;351:939–43.
Robert T, Nore A, Brun C, Maffre C, Crimi B, Bourbon HM, et al. The TopoVIB-Like protein family is required for meiotic DNA double-strand break formation. Science. 2016;351:943–9.
Keeney S, Lange J, Mohibullah N. Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu Rev Genet. 2014;48:187–214.
Boekhout M, Karasu ME, Wang J, Acquaviva L, Pratto F, Brick K, et al. REC114 Partner ANKRD31 Controls Number, Timing, and Location of Meiotic DNA Breaks. Mol Cell. 2019;74:1053–68.e8.
Acquaviva L, Boekhout M, Karasu ME, Brick K, Pratto F, Li T, et al. Ensuring meiotic DNA break formation in the mouse pseudoautosomal region. Nature. 2020;582:426–31.
Smirnova NA, Romanienko PJ, Khil PP, Camerini-Otero RD. Gene expression profiles of Spo11-/- mouse testes with spermatocytes arrested in meiotic prophase I. Reproduction. 2006;132:67–77.
Acknowledgements
We would like to thank Yan Hong, Wei Chen, Xiaobo Wang, Cunzhong Deng, Hongfang Sun, and Jianxiong Zhang for the coordinated study recruitment and sample collection.
Funding
This work was supported by National Natural Science Foundation of China (82001530, 81903417, 81871215, and 81971368), Clinical Research Innovation Plan of Shanghai General Hospital (KD007-ly01, CTCCR-C04), Clinical Research Plan of SHDC (SHDC2020CR3077B), and the Key Project of Research and Development of Ningxia Hui Autonomous Region of China (2020BFH02002), and Shanghai Sailing Program (20YF1439500 and 20YF1453700).
Author information
Authors and Affiliations
Contributions
Conceptualization: YXZ, NL, ZYJ, CCY, ZL and YCZ; Data curation: HWB, RHT, PL and JPZ; Formal Analysis: ELZ, YHH, JPZ and YQH; Funding acquisition: CCY, ZL and YCZ; Investigation: NJO, HWB and JPZ; Project administration: YXZ, NL and ZYJ, Supervision: RHT, YQH and JZ; Visualization: PL and YHH; Writing – original draft: YXZ and NL; Writing – review & editing: YXZ, CCY, ZL, YCZ and JZ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Ethical Review Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine (Permit Number 2020SQ199). Written informed consent was obtained from the patient and his parents for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Li, N., Ji, Z. et al. Bi-allelic MEI1 variants cause meiosis arrest and non-obstructive azoospermia. J Hum Genet 68, 383–392 (2023). https://doi.org/10.1038/s10038-023-01119-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-023-01119-3
This article is cited by
-
Identification of genomic characteristics and selective signals in Guizhou black goat
BMC Genomics (2024)
-
Novel MEI1 mutations cause chromosomal and DNA methylation abnormalities leading to embryonic arrest and implantation failure
Molecular Genetics and Genomics (2024)