Abstract
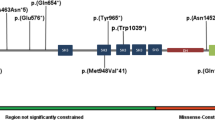
Kinesin Family Member 21B (KIF21B) encoded by KIF21B (MIM*608322), belongs to the Kinesin superfamily proteins, which play a key role in microtubule organisation in neuronal dendrites and axons. Recently, heterozygous variants in KIF21B were implicated as the cause of intellectual disability and brain malformations in four unrelated individuals. We report a 9-year-old male with delayed speech, hyperactivity, poor social interaction, and autistic features. A parent-offspring trio exome sequencing identified a novel de novo rare heterozygous variant, NM_001252102.2: c.1513A>C, p.(Ser505Arg) in exon 11 of KIF21B. In vivo functional analysis using in utero electroporation in mouse embryonic cortex revealed that the expression of Ser505Arg KIF21B protein in the cerebral cortex impaired the radial migration of projection neurons, thus confirming the pathogenicity of the variant. Our report further validates pathogenic variants in KIF21B as a cause of neurodevelopmental disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–26.
Marszalek JR, Weiner JA, Farlow SJ, Chun J, Goldstein LS. Novel dendritic kinesin sorting identified by different process targeting of two related kinesins: KIF21A and KIF21B. J Cell Biol. 1999;145:469–79.
Ghiretti AE, Thies E, Tokito MK, Lin T, Ostap EM, Kneussel M, et al. Activity-dependent regulation of distinct transport and cytoskeletal remodeling functions of the dendritic kinesin KIF21B. Neuron. 2016;92:857–72.
Muhia M, Thies E, Labonté D, Ghiretti AE, Gromova KV, Xompero F, et al. The kinesin KIF21B regulates microtubule dynamics and is essential for neuronal morphology, synapse function, and learning and memory. Cell Rep. 2016;15:968–77.
Kannan M, Bayam E, Wagner C, Rinaldi B, Kretz PF, Tilly P, et al. WD40-repeat 47, a microtubule-associated protein, is essential for brain development and autophagy. Proc Natl Acad Sci USA. 2017;114:E9308–17.
Morikawa M, Tanaka Y, Cho HS, Yoshihara M, Hirokawa N. The molecular motor KIF21B mediates synaptic plasticity and fear extinction by terminating Rac1 activation. Cell Rep. 2018;23:3864–77.
Asselin L, Rivera Alvarez J, Heide S, Bonnet CS, Tilly P, Vitet H, et al. Mutations in the KIF21B kinesin gene cause neurodevelopmental disorders through imbalanced canonical motor activity. Nat Commun. 2020;11:2441.
Girisha KM, von Elsner L, Neethukrishna K, Muranjan M, Shukla A, Bhavani GS, et al. The homozygous variant c.797G>A/p.(Cys266Tyr) in PISD is associated with a Spondyloepimetaphyseal dysplasia with large epiphyses and disturbed mitochondrial function. Hum Mutat. 2019;40:299–309.
Acknowledgements
We thank the family for their co-operation. This work was funded by grants from INSERM (ATIP-Avenir programme, JDG), the French state funds through the Agence Nationale de la Recherche under the project JCJC CREDO ANR-14-CE13-0008-01 (JDG) and the programme Investissements d’Avenir labelled (ANR-10-IDEX-0002-02, ANR-10-LABX-0030-INRT, to JDG), INSERM/CNRS and University of Strasbourg. This work of the Interdisciplinary Thematic Institute IMCBio, as part of the ITI 2021-2028 programme of the University of Strasbourg, CNRS and Inserm, was supported by IdEx Unistra (ANR-10-IDEX-0002), and by SFRI-STRAT’US project (ANR 20-SFRI-0012) and EUR IMCBio (ANR-17-EURE-0023) under the framework of the French Investments for the Future Program. JRA was funded through the IGBMC PhD programme (ANR-10-IDEX-0002-02, ANR-10-LABX-0030-INRT). JRA is currently supported by the Fondation pour la recherche médicale. PT is a research assistant at the University of Strasbourg. JDG is INSERM investigator. We are grateful to the mouse facility of the Institut Clinique de la Souris (ICS) for taking care of the mice, to the Molecular Biology service of the IGBMC (in particular Thierry Lerouge) for their collaboration in making the plasmids used in this study and to Noémie Schwaller for her technical help. We acknowledge the National Institutes of Health, United States for funding the study titled, ‘Genetic Diagnosis of Neurodevelopmental Disorders in India’ (1R01HD093570-01A1). DLN is a DBT/Wellcome Trust India Alliance Early Career Clinical and Public Health Research Fellow.
Author information
Authors and Affiliations
Contributions
DLN collected clinical data, and drafted the manuscript. JRA performed the experiments on mice and analyzed the data. PT performed experiments on mice. MCdR did in-silico modeling. VB was involved in collecting clinical information. JDG and AS supervised the work and design of research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Narayanan, D.L., Rivera Alvarez, J., Tilly, P. et al. Further delineation of KIF21B-related neurodevelopmental disorders. J Hum Genet 67, 729–733 (2022). https://doi.org/10.1038/s10038-022-01087-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-022-01087-0
This article is cited by
-
Recent advances in novel mutation genes of Parkinson's disease
Journal of Neurology (2023)
-
De novo variants underlying monogenic syndromes with intellectual disability in a neurodevelopmental cohort from India
European Journal of Human Genetics (2023)