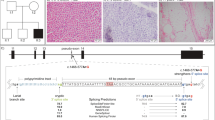
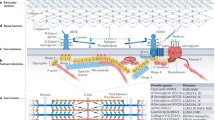
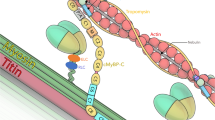
Abstract
In this review, we focus on congenital myopathies, which are a genetically heterogeneous group of hereditary muscle diseases with slow or minimal progression. They are mainly defined and classified according to pathological features, with the major subtypes being core myopathy (central core disease), nemaline myopathy, myotubular/centronuclear myopathy, and congenital fiber-type disproportion myopathy. Recent advances in molecular genetics, especially next-generation sequencing technology, have rapidly increased the number of known causative genes for congenital myopathies; however, most of the diseases related to the novel causative genes are extremely rare. There remains no cure for congenital myopathies. However, there have been recent promising findings that could inform the development of therapy for several types of congenital myopathies, including myotubular myopathy, which indicates the importance of prompt and correct diagnosis. This review discusses the major causative genes (NEB, ACTA1, ADSSL1, RYR1, SELENON, MTM1, DNM2, and TPM3) for each subtype of congenital myopathies and the relevant latest findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jungbluth H, Treves S, Zorzato F, Sarkozy A, Ochala J, Sewry C, et al. Congenital myopathies: disorders of excitation-contraction coupling and muscle contraction. Nat Rev Neurol. 2018;14:151–67.
North KN, Wang CH, Clarke N, Jungbluth H, Vainzof M, Dowling JJ, et al. Approach to the diagnosis of congenital myopathies. Neuromuscul Disord. 2014;24:97–116.
Jungbluth H, Muntoni F. Therapeutic aspects in congenital myopathies. Semin Pediatr Neurol. 2019;29:71–82.
Laitila J, Wallgren-Pettersson C. Recent advances in nemaline myopathy. Neuromuscul Disord. 2021;31:955–67.
Saito Y, Nishikawa A, Iida A, Mori-Yoshimura M, Oya Y, Ishiyama A, et al. ADSSL1 myopathy is the most common nemaline myopathy in Japan with variable clinical features. Neurology. 2020;95:e1500-11.
Nilipour Y, Nafissi S, Tjust AE, Ravenscroft G, Hossein Nejad Nedai H, Taylor RL, et al. Ryanodine receptor type 3 (RYR3) as a novel gene associated with a myopathy with nemaline bodies. Eur J Neurol. 2018;25:841–7.
Sewry CA, Laitila JM, Wallgren-Pettersson C. Nemaline myopathies: a current view. J Muscle Res Cell Motil. 2019;40:111–26.
Zukosky K, Meilleur K, Traynor BJ, Dastgir J, Medne L, Devoto M, et al. Association of a novel ACTA1 mutation with a dominant progressive scapuloperoneal myopathy in an extended family. JAMA Neurol. 2015;72:689–98.
Nowak KJ, Ravenscroft G, Laing NG. Skeletal muscle α-actin diseases (actinopathies): pathology and mechanisms. Acta Neuropathol. 2013;125:19–32.
Ogasawara M, Nishino I. A review of core myopathy: central core disease, multiminicore disease, dusty core disease, and core-rod myopathy. Neuromuscul Disord. 2021;31:968–77.
Shy GM, Engel WK, Somers JE, Wanko T. Nemaline myopathy. A new congenital myopathy. Brain. 1963;86:793–810.
Conen PE, Murphy EG, Donohue WL. Light and electron microscopic studies of “myogranules” in a child with hypotonia and muscle weakness. Can Med Assoc J. 1963;89:983–6.
Malfatti E, Lehtokari VL, Böhm J, De Winter JM, Schäffer U, Estournet B, et al. Muscle histopathology in nebulin-related nemaline myopathy: ultrastrastructural findings correlated to disease severity and genotype. Acta Neuropathol Commun. 2014;2:44.
Pelin K, Hilpelä P, Donner K, Sewry C, Akkari PA, Wilton SD, et al. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA. 1999;96:2305–10.
Nowak KJ, Wattanasirichaigoon D, Goebel HH, Wilce M, Pelin K, Donner K, et al. Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet. 1999;23:208–12.
Amburgey K, Acker M, Saeed S, Amin R, Beggs AH, Bönnemann CG, et al. A cross-sectional study of nemaline myopathy. Neurology. 2021;96:e1425–36.
Wang Q, Hu Z, Chang X, Yu M, Xie Z, Lv H, et al. Mutational and clinical spectrum in a cohort of Chinese patients with hereditary nemaline myopathy. Clin Genet. 2020;97:878–89.
Chu M, Gregorio CC, Pappas CT. Nebulin, a multi-functional giant. J Exp Biol. 2016;219:146–52.
Lehtokari VL, Kiiski K, Sandaradura SA, Laporte J, Repo P, Frey JA, et al. Mutation update: the spectra of nebulin variants and associated myopathies. Hum Mutat. 2014;35:1418–26.
Kiiski KJ, Lehtokari VL, Vihola AK, Laitila JM, Huovinen S, Sagath LJ, et al. Dominantly inherited distal nemaline/cap myopathy caused by a large deletion in the nebulin gene. Neuromuscul Disord. 2019;29:97–107.
Wallgren-Pettersson C, Pelin K, Nowak KJ, Muntoni F, Romero NB, Goebel HH, et al. Genotype-phenotype correlations in nemaline myopathy caused by mutations in the genes for nebulin and skeletal muscle alpha-actin. Neuromuscul Disord. 2004;14:461–70.
Park YE, Shin JH, Kim HS, Lee CH, Kim DS. Characterization of congenital myopathies at a Korean neuromuscular center. Muscle Nerve. 2018;58:235–44.
Colombo I, Scoto M, Manzur AY, Robb SA, Maggi L, Gowda V, et al. Congenital myopathies: natural history of a large pediatric cohort. Neurology. 2015;84:28–35.
Wang CH, Dowling JJ, North K, Schroth MK, Sejersen T, Shapiro F, et al. Consensus statement on standard of care for congenital myopathies. J Child Neurol. 2012;27:363–82.
Lehtokari VL, Pelin K, Sandbacka M, Ranta S, Donner K, Muntoni F, et al. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum Mutat. 2006;27:946–56.
Romero NB, Lehtokari VL, Quijano-Roy S, Monnier N, Claeys KG, Carlier RY, et al. Core-rod myopathy caused by mutations in the nebulin gene. Neurology. 2009;73:1159–61.
Scacheri PC, Hoffman EP, Fratkin JD, Semino-Mora C, Senchak A, Davis MR, et al. A novel ryanodine receptor gene mutation causing both cores and rods in congenital myopathy. Neurology. 2000;55:1689–96.
Jungbluth H, Sewry CA, Counsell S, Allsop J, Chattopadhyay A, Mercuri E, et al. Magnetic resonance imaging of muscle in nemaline myopathy. Neuromuscul Disord. 2004;14:779–84.
Laing NG, Dye DE, Wallgren-Pettersson C, Richard G, Monnier N, Lillis S, et al. Mutations and polymorphisms of the skeletal muscle alpha-actin gene (ACTA1). Hum Mutat. 2009;30:1267–77.
de Winter JM, Ottenheijm CAC. Sarcomere dysfunction in nemaline myopathy. J Neuromuscul Dis. 2017;4:99–113.
Sparrow JC, Nowak KJ, Durling HJ, Beggs AH, Wallgren-Pettersson C, Romero N, et al. Muscle disease caused by mutations in the skeletal muscle alpha-actin gene (ACTA1). Neuromuscul Disord. 2003;13:519–31.
Agrawal PB, Strickland CD, Midgett C, Morales A, Newburger DE, Poulos MA, et al. Heterogeneity of nemaline myopathy cases with skeletal muscle alpha-actin gene mutations. Ann Neurol. 2004;56:86–96.
Goebel HH, Anderson JR, Hübner C, Oexle K, Warlo I. Congenital myopathy with excess of thin myofilaments. Neuromuscul Disord. 1997;7:160–8.
Jungbluth H, Sewry CA, Brown SC, Nowak KJ, Laing NG, Wallgren-Pettersson C, et al. Mild phenotype of nemaline myopathy with sleep hypoventilation due to a mutation in the skeletal muscle alpha-actin (ACTA1) gene. Neuromuscul Disord. 2001;11:35–40.
Kaindl AM, Rüschendorf F, Krause S, Goebel HH, Koehler K, Becker C, et al. Missense mutations of ACTA1 cause dominant congenital myopathy with cores. J Med Genet. 2004;41:842–8.
Garibaldi M, Fattori F, Pennisi EM, Merlonghi G, Fionda L, Vanoli F, et al. Novel ACTA1 mutation causes late-presenting nemaline myopathy with unusual dark cores. Neuromuscul Disord. 2021;31:139–48.
Hung RM, Yoon G, Hawkins CE, Halliday W, Biggar D, Vajsar J. Cap myopathy caused by a mutation of the skeletal alpha-actin gene ACTA1. Neuromuscul Disord. 2010;20:238–40.
Laing NG, Clarke NF, Dye DE, Liyanage K, Walker KR, Kobayashi Y, et al. Actin mutations are one cause of congenital fibre type disproportion. Ann Neurol. 2004;56:689–94.
Sewry CA, Holton JL, Dick DJ, Muntoni F, Hanna MG. Zebra body myopathy is caused by a mutation in the skeletal muscle actin gene (ACTA1). Neuromuscul Disord. 2015;25:388–91.
Castiglioni C, Cassandrini D, Fattori F, Bellacchio E, D’Amico A, Alvarez K, et al. Muscle magnetic resonance imaging and histopathology in ACTA1-related congenital nemaline myopathy. Muscle Nerve. 2014;50:1011–6.
Quijano-Roy S, Carlier RY, Fischer D. Muscle imaging in congenital myopathies. Semin Pediatr Neurol. 2011;18:221–9.
Park HJ, Hong YB, Choi YC, Lee J, Kim EJ, Lee JS, et al. ADSSL1 mutation relevant to autosomal recessive adolescent onset distal myopathy. Ann Neurol. 2016;79:231–43.
Lipps G, Krauss G. Adenylosuccinate synthase from Saccharomyces cerevisiae: homologous overexpression, purification and characterization of the recombinant protein. Biochem J. 1999;341(Pt 3):537–43.
Park HJ, Shin HY, Kim S, Kim SH, Lee Y, Lee JH, et al. Distal myopathy with ADSSL1 mutations in Korean patients. Neuromuscul Disord. 2017;27:465–72.
Mroczek M, Durmus H, Bijarnia-Mahay S, Töpf A, Ghaoui R, Bryen S, et al. Expanding the disease phenotype of ADSSL1-associated myopathy in non-Korean patients. Neuromuscul Disord. 2020;30:310–4.
Magee KR, Shy GM. A new congenital non-progressive myopathy. Brain. 1956;79:610–21.
Dubowitz V, Pearse AG. Oxidative enzymes and phosphorylase in central-core disease of muscle. Lancet. 1960;2:23–4.
Engel AG, Gomez MR, Groover RV. Multicore disease. A recently recognized congenital myopathy associated with multifocal degeneration of muscle fibers. Mayo Clin Proc. 1971;46:666–81.
Sewry CA, Wallgren-Pettersson C. Myopathology in congenital myopathies. Neuropathol Appl Neurobiol. 2017;43:5–23.
Wu S, Ibarra MC, Malicdan MC, Murayama K, Ichihara Y, Kikuchi H, et al. Central core disease is due to RYR1 mutations in more than 90% of patients. Brain. 2006;129:1470–80.
Ferreiro A, Quijano-Roy S, Pichereau C, Moghadaszadeh B, Goemans N, Bönnemann C, et al. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet. 2002;71:739–49.
Witherspoon JW, Meilleur KG. Review of RyR1 pathway and associated pathomechanisms. Acta Neuropathol Commun. 2016;4:121.
Todd JJ, Sagar V, Lawal TA, Allen C, Razaqyar MS, Shelton MS, et al. Correlation of phenotype with genotype and protein structure in RYR1-related disorders. J Neurol. 2018;265:2506–24.
Quane KA, Healy JM, Keating KE, Manning BM, Couch FJ, Palmucci LM, et al. Mutations in the ryanodine receptor gene in central core disease and malignant hyperthermia. Nat Genet. 1993;5:51–5.
Klein A, Lillis S, Munteanu I, Scoto M, Zhou H, Quinlivan R, et al. Clinical and genetic findings in a large cohort of patients with ryanodine receptor 1 gene-associated myopathies. Hum Mutat. 2012;33:981–8.
Clarke NF. Congenital fibre type disproportion—a syndrome at the crossroads of the congenital myopathies. Neuromuscul Disord. 2011;21:252–3.
Clarke NF, Waddell LB, Cooper ST, Perry M, Smith RL, Kornberg AJ, et al. Recessive mutations in RYR1 are a common cause of congenital fiber type disproportion. Hum Mutat. 2010;31:E1544–50.
Sato I, Wu S, Ibarra MC, Hayashi YK, Fujita H, Tojo M, et al. Congenital neuromuscular disease with uniform type 1 fiber and RYR1 mutation. Neurology. 2008;70:114–22.
Garibaldi M, Rendu J, Brocard J, Lacene E, Faure J, Brochier G, et al. ‘Dusty core disease’ (DuCD): expanding morphological spectrum of RYR1 recessive myopathies. Acta Neuropathol Commun. 2019;7:3.
Ogasawara M, Ogawa M, Nonaka I, Hayashi S, Noguchi S, Nishino I. Evaluation of the core formation process in congenital neuromuscular disease with uniform type 1 fiber and central core disease. J Neuropathol Exp Neurol. 2020;79:1370–5.
von der Hagen M, Kress W, Hahn G, Brocke KS, Mitzscherling P, Huebner A, et al. Novel RYR1 missense mutation causes core rod myopathy. Eur J Neurol. 2008;15:e31–2.
Wilmshurst JM, Lillis S, Zhou H, Pillay K, Henderson H, Kress W, et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol. 2010;68:717–26.
Amburgey K, McNamara N, Bennett LR, McCormick ME, Acsadi G, Dowling JJ. Prevalence of congenital myopathies in a representative pediatric united states population. Ann Neurol. 2011;70:662–5.
Manzur AY, Sewry CA, Ziprin J, Dubowitz V, Muntoni F. A severe clinical and pathological variant of central core disease with possible autosomal recessive inheritance. Neuromuscul Disord. 1998;8:467–73.
Patterson VH, Hill TR, Fletcher PJ, Heron JR. Central core disease: clinical and pathological evidence of progression within a family. Brain. 1979;102:581–94.
Tojo M, Ozawa M, Nonaka I. Central core disease and congenital neuromuscular disease with uniform type 1 fibers in one family. Brain Dev. 2000;22:262–4.
Oh SJ, Danon MJ. Nonprogressive congenital neuromuscular disease with uniform type 1 fiber. Arch Neurol. 1983;40:147–50.
Sewry CA, Muller C, Davis M, Dwyer JS, Dove J, Evans G, et al. The spectrum of pathology in central core disease. Neuromuscul Disord. 2002;12:930–8.
Klein A, Jungbluth H, Clement E, Lillis S, Abbs S, Munot P, et al. Muscle magnetic resonance imaging in congenital myopathies due to ryanodine receptor type 1 gene mutations. Arch Neurol. 2011;68:1171–9.
Straub V, Carlier PG, Mercuri E. TREAT-NMD workshop: pattern recognition in genetic muscle diseases using muscle MRI: 25-26 February 2011, Rome, Italy. Neuromuscul Disord. 2012;22:S42–53.
Dowling JJ, Arbogast S, Hur J, Nelson DD, McEvoy A, Waugh T, et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain. 2012;135:1115–27.
Todd JJ, Lawal TA, Witherspoon JW, Chrismer IC, Razaqyar MS, Punjabi M, et al. Randomized controlled trial of N-acetylcysteine therapy for RYR1-related myopathies. Neurology. 2020;94:e1434–44.
Marino M, Stoilova T, Giorgi C, Bachi A, Cattaneo A, Auricchio A, et al. SEPN1, an endoplasmic reticulum-localized selenoprotein linked to skeletal muscle pathology, counteracts hyperoxidation by means of redox-regulating SERCA2 pump activity. Hum Mol Genet. 2015;24:1843–55.
Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Quijano Roy S, Merlini L, et al. Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet. 2001;29:17–8.
Villar-Quiles RN, von der Hagen M, Métay C, Gonzalez V, Donkervoort S, Bertini E, et al. The clinical, histologic, and genotypic spectrum of SEPN1-related myopathy: a case series. Neurology. 2020;95:e1512–27.
Silwal A, Sarkozy A, Scoto M, Ridout D, Schmidt A, Laverty A, et al. Selenoprotein N-related myopathy: a retrospective natural history study to guide clinical trials. Ann Clin Transl Neurol. 2020;7:2288–96.
Ferreiro A, Ceuterick-de Groote C, Marks JJ, Goemans N, Schreiber G, Hanefeld F, et al. Desmin-related myopathy with Mallory body-like inclusions is caused by mutations of the selenoprotein N gene. Ann Neurol. 2004;55:676–86.
Clarke NF, Kidson W, Quijano-Roy S, Estournet B, Ferreiro A, Guicheney P, et al. SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann Neurol. 2006;59:546–52.
Mercuri E, Clements E, Offiah A, Pichiecchio A, Vasco G, Bianco F, et al. Muscle magnetic resonance imaging involvement in muscular dystrophies with rigidity of the spine. Ann Neurol. 2010;67:201–8.
Spiro AJ, Shy GM, Gonatas NK. Myotubular myopathy. Persistence of fetal muscle in an adolescent boy. Arch Neurol. 1966;14:1–14.
Sher JH, Rimalovski AB, Athanassiades TJ, Aronson SM. Familial centronuclear myopathy: a clinical and pathological study. Neurology. 1967;17:727–42.
Romero NB. Centronuclear myopathies: a widening concept. Neuromuscul Disord. 2010;20:223–8.
Hamanaka K, Inami I, Wada T, Mitsuhashi S, Noguchi S, Hayashi YK, et al. Muscle from a 20-week-old myotubular myopathy fetus is not myotubular. Neuromuscul Disord. 2016;26:234–5.
Bitoun M, Maugenre S, Jeannet PY, Lacène E, Ferrer X, Laforêt P, et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet. 2005;37:1207–9.
Nicot AS, Toussaint A, Tosch V, Kretz C, Wallgren-Pettersson C, Iwarsson E, et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet. 2007;39:1134–9.
Ceyhan-Birsoy O, Agrawal PB, Hidalgo C, Schmitz-Abe K, DeChene ET, Swanson LC, et al. Recessive truncating titin gene, TTN, mutations presenting as centronuclear myopathy. Neurology. 2013;81:1205–14.
Agrawal PB, Pierson CR, Joshi M, Liu X, Ravenscroft G, Moghadaszadeh B, et al. SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am J Hum Genet. 2014;95:218–26.
Vandersmissen I, Biancalana V, Servais L, Dowling JJ, Vander Stichele G, Van, et al. An integrated modelling methodology for estimating the prevalence of centronuclear myopathy. Neuromuscul Disord. 2018;28:766–77.
Reumers SFI, Erasmus CE, Bouman K, Pennings M, Schouten M, Kusters B, et al. Clinical, genetic, and histological features of centronuclear myopathy in the Netherlands. Clin Genet. 2021;100:692–702.
Noguchi S, Fujita M, Murayama K, Kurokawa R, Nishino I. Gene expression analyses in X-linked myotubular myopathy. Neurology. 2005;65:732–7.
Robinson FL, Dixon JE. Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol. 2006;16:403–12.
Hnia K, Vaccari I, Bolino A, Laporte J. Myotubularin phosphoinositide phosphatases: cellular functions and disease pathophysiology. Trends Mol Med. 2012;18:317–27.
Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, Coy JF, et al. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet. 1996;13:175–82.
Biancalana V, Beggs AH, Das S, Jungbluth H, Kress W, Nishino I, et al. Clinical utility gene card for: centronuclear and myotubular myopathies. Eur J Hum Genet. 2012;20.
Biancalana V, Scheidecker S, Miguet M, Laquerrière A, Romero NB, Stojkovic T, et al. Affected female carriers of MTM1 mutations display a wide spectrum of clinical and pathological involvement: delineating diagnostic clues. Acta Neuropathol. 2017;134:889–904.
Lawlor MW, Dowling JJ. X-linked myotubular myopathy. Neuromuscul Disord. 2021;31:1004–12.
McEntagart M, Parsons G, Buj-Bello A, Biancalana V, Fenton I, Little M, et al. Genotype-phenotype correlations in X-linked myotubular myopathy. Neuromuscul Disord. 2002;12:939–46.
Annoussamy M, Lilien C, Gidaro T, Gargaun E, Chê V, Schara U, et al. X-linked myotubular myopathy: a prospective international natural history study. Neurology. 2019;92:e1852–67.
Cocanougher BT, Flynn L, Yun P, Jain M, Waite M, Vasavada R, et al. Adult MTM1-related myopathy carriers: classification based on deep phenotyping. Neurology. 2019;93:e1535–42.
Lawlor MW, Beggs AH, Buj-Bello A, Childers MK, Dowling JJ, James ES, et al. Skeletal muscle pathology in X-linked myotubular myopathy: review with cross-species comparisons. J Neuropathol Exp Neurol. 2016;75:102–10.
Bevilacqua JA, Bitoun M, Biancalana V, Oldfors A, Stoltenburg G, Claeys KG, et al. “Necklace” fibers, a new histological marker of late-onset MTM1-related centronuclear myopathy. Acta Neuropathol. 2009;117:283–91.
Dubowitz V, Sewry CA, Oldfors A. Muscle biopsy: a practical approach. 5th edn. Saunders Elsevier, Printed in China, pp 1–530. 2020.
Cowling BS, Chevremont T, Prokic I, Kretz C, Ferry A, Coirault C, et al. Reducing dynamin 2 expression rescues X-linked centronuclear myopathy. J Clin Invest. 2014;124:1350–63.
Maani N, Sabha N, Rezai K, Ramani A, Groom L, Eltayeb N, et al. Tamoxifen therapy in a murine model of myotubular myopathy. Nat Commun. 2018;9:4849.
Gayi E, Neff LA, Massana Muñoz X, Ismail HM, Sierra M, Mercier T, et al. Tamoxifen prolongs survival and alleviates symptoms in mice with fatal X-linked myotubular myopathy. Nat Commun. 2018;9:4848.
Böhm J, Biancalana V, Dechene ET, Bitoun M, Pierson CR, Schaefer E, et al. Mutation spectrum in the large GTPase dynamin 2, and genotype-phenotype correlation in autosomal dominant centronuclear myopathy. Hum Mutat. 2012;33:949–59.
Gu C, Yaddanapudi S, Weins A, Osborn T, Reiser J, Pollak M, et al. Direct dynamin-actin interactions regulate the actin cytoskeleton. Embo J. 2010;29:3593–606.
Fujise K, Okubo M, Abe T, Yamada H, Nishino I, Noguchi S, et al. Mutant BIN1-Dynamin 2 complexes dysregulate membrane remodeling in the pathogenesis of centronuclear myopathy. J Biol Chem. 2021;296:100077.
Kenniston JA, Lemmon MA. Dynamin GTPase regulation is altered by PH domain mutations found in centronuclear myopathy patients. Embo J. 2010;29:3054–67.
Gómez-Oca R, Cowling BS, Laporte J. Common pathogenic mechanisms in centronuclear and myotubular myopathies and latest treatment advances. Int J Mol Sci. 2021;22.
Wang L, Barylko B, Byers C, Ross JA, Jameson DM, Albanesi JP. Dynamin 2 mutants linked to centronuclear myopathies form abnormally stable polymers. J Biol Chem. 2010;285:22753–7.
Demonbreun AR, McNally EM. Dynamin 2 the rescue for centronuclear myopathy. J Clin Invest. 2014;124:976–8.
Bitoun M, Bevilacqua JA, Prudhon B, Maugenre S, Taratuto AL, Monges S, et al. Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol. 2007;62:666–70.
Catteruccia M, Fattori F, Codemo V, Ruggiero L, Maggi L, Tasca G, et al. Centronuclear myopathy related to dynamin 2 mutations: clinical, morphological, muscle imaging and genetic features of an Italian cohort. Neuromuscul Disord. 2013;23:229–38.
Jungbluth H, Gautel M. Pathogenic mechanisms in centronuclear myopathies. Front Aging Neurosci. 2014;6:339.
Fischer D, Herasse M, Bitoun M, Barragán-Campos HM, Chiras J, Laforêt P, et al. Characterization of the muscle involvement in dynamin 2-related centronuclear myopathy. Brain. 2006;129:1463–9.
Biancalana V, Romero NB, Thuestad IJ, Ignatius J, Kataja J, Gardberg M, et al. Some DNM2 mutations cause extremely severe congenital myopathy and phenocopy myotubular myopathy. Acta Neuropathol Commun. 2018;6:93.
Buono S, Ross JA, Tasfaout H, Levy Y, Kretz C, Tayefeh L, et al. Reducing dynamin 2 (DNM2) rescues DNM2-related dominant centronuclear myopathy. Proc Natl Acad Sci USA. 2018;115:11066–71.
Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types. 4. Children’s biopsies. Neurology. 1969;19:591–605.
Clarke NF, North KN. Congenital fiber type disproportion-30 years on. J Neuropathol Exp Neurol. 2003;62:977–89.
Brooke MH. Congenital fiber type disproportion. In: Kakulas BA, editor. Clinical Studies in Myology: Proceedings of the 2nd International Congress on Muscle Diseases, Perth, Australia, Nov. 22–29, 1971. Amsterdam: Excerpta Medica; 1973. p. 147–59.
Clarke NF. Congenital fiber-type disproportion. Semin Pediatr Neurol. 2011;18:264–71.
Claeys KG. Congenital myopathies: an update. Dev Med Child Neurol. 2020;62:297–302.
Clarke NF, Kolski H, Dye DE, Lim E, Smith RL, Patel R, et al. Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol. 2008;63:329–37.
Lawlor MW, Dechene ET, Roumm E, Geggel AS, Moghadaszadeh B, Beggs AH. Mutations of tropomyosin 3 (TPM3) are common and associated with type 1 myofiber hypotrophy in congenital fiber type disproportion. Hum Mutat. 2010;31:176–83.
Donkervoort S, Papadaki M, de Winter JM, Neu MB, Kirschner J, Bolduc V, et al. TPM3 deletions cause a hypercontractile congenital muscle stiffness phenotype. Ann Neurol. 2015;78:982–94.
Laing NG, Wilton SD, Akkari PA, Dorosz S, Boundy K, Kneebone C, et al. A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy. Nat Genet. 1995;9:75–9.
Tan P, Briner J, Boltshauser E, Davis MR, Wilton SD, North K, et al. Homozygosity for a nonsense mutation in the alpha-tropomyosin slow gene TPM3 in a patient with severe infantile nemaline myopathy. Neuromuscul Disord. 1999;9:573–9.
Kiphuth IC, Krause S, Huttner HB, Dekomien G, Struffert T, Schröder R. Autosomal dominant nemaline myopathy caused by a novel alpha-tropomyosin 3 mutation. J Neurol. 2010;257:658–60.
Schreckenbach T, Schröder JM, Voit T, Abicht A, Neuen-Jacob E, Roos A, et al. Novel TPM3 mutation in a family with cap myopathy and review of the literature. Neuromuscul Disord. 2014;24:117–24.
Hsu PJ, Wang HD, Tseng YC, Pan SW, Sampurna BP, Jong YJ, et al. L-Carnitine ameliorates congenital myopathy in a tropomyosin 3 de novo mutation transgenic zebrafish. J Biomed Sci. 2021;28:8.
Liewluck T, Milone M, Tian X, Engel AG, Staff NP, Wong LJ. Adult-onset respiratory insufficiency, scoliosis, and distal joint hyperlaxity in patients with multiminicore disease due to novel Megf10 mutations. Muscle Nerve. 2016;53:984–8.
Dafsari HS, Kocaturk NM, Daimagüler HS, Brunn A, Dötsch J, Weis J, et al. Bi-allelic mutations in uncoordinated mutant number-45 myosin chaperone B are a cause for congenital myopathy. Acta Neuropathol Commun. 2019;7:211.
Donkervoort S, Kutzner CE, Hu Y, Lornage X, Rendu J, Stojkovic T, et al. Pathogenic variants in the myosin chaperone UNC-45B cause progressive myopathy with eccentric cores. Am J Hum Genet. 2020;107:1078–95.
Majczenko K, Davidson AE, Camelo-Piragua S, Agrawal PB, Manfready RA, Li X, et al. Dominant mutation of CCDC78 in a unique congenital myopathy with prominent internal nuclei and atypical cores. Am J Hum Genet. 2012;91:365–71.
Tajsharghi H, Oldfors A. Myosinopathies: pathology and mechanisms. Acta Neuropathol. 2013;125:3–18.
Wallgren-Pettersson C, Lehtokari VL, Kalimo H, Paetau A, Nuutinen E, Hackman P, et al. Distal myopathy caused by homozygous missense mutations in the nebulin gene. Brain. 2007;130:1465–76.
Jungbluth H. Central core disease. Orphanet J Rare Dis. 2007;2:25.
Herman GE, Finegold M, Zhao W, de Gouyon B, Metzenberg A. Medical complications in long-term survivors with X-linked myotubular myopathy. J Pediatr. 1999;134:206–14.
Beggs AH, Byrne BJ, De Chastonay S, Haselkorn T, Hughes I, James ES, et al. A multicenter, retrospective medical record review of X-linked myotubular myopathy: the recensus study. Muscle Nerve. 2018;57:550–60.
Amburgey K, Tsuchiya E, de Chastonay S, Glueck M, Alverez R, Nguyen CT, et al. A natural history study of X-linked myotubular myopathy. Neurology. 2017;89:1355–64.
Abath Neto O, Silva MR, Martins Cde A, Oliveira Ade S, Reed UC, Biancalana V, et al. A study of a cohort of X-linked myotubular myopathy at the clinical, histologic, and genetic levels. Pediatr Neurol. 2016;58:107–12.
Acknowledgements
We thank Ms. Kaoru Tatezawa, Ms. Kazu Iwasawa, Ms. Yoko Tsutsumi, and Ms. Kanae Kanna, Mr. Hisayoshi Nakamura in NCNP for their technical assistance.
Funding
This study was supported partly by Intramural Research Grant (2-5) for Neurological and Psychiatric Disorders of NCNP and AMED under Grant Number JP21ek0109490h0002.
Author information
Authors and Affiliations
Contributions
M.O. designed the study. M.O. and I.N. wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All patients provided informed consent for using their samples for research after the diagnosis. This study was approved by the ethical committees of the NCNP (A2019-123).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogasawara, M., Nishino, I. A review of major causative genes in congenital myopathies. J Hum Genet 68, 215–225 (2023). https://doi.org/10.1038/s10038-022-01045-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-022-01045-w
This article is cited by
-
Speg interactions that regulate the stability of excitation-contraction coupling protein complexes in triads and dyads
Communications Biology (2023)