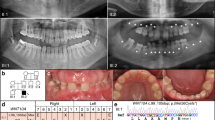
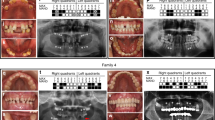
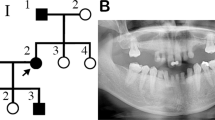
Abstract
Tooth agenesis is one of the most frequent congenital abnormalities found in the maxillofacial region. Oligodontia, a severe form of tooth agenesis, occurs as an isolated anomaly or as a syndromic feature. We performed whole exome sequencing analyses to identify causative mutation in a Japanese family with three affected individuals with non-syndromic oligodontia. After variant filtering procedures and validation by Sanger sequencing, we identified one missense mutation (c.668 C > T, p.Gly223Asp) in OPN3 at 1q43, encoding a photosensitive G-protein-coupled receptor (GPCR) expressed in various tissues including brain, liver, and adipose. This mutation was predicted to be pathogenic in silico and was not found in the public databases. We further examined 48 genetically unrelated cases by targeted sequencing of the OPN3 gene region and found one additional missense variant in this gene (c.768 C > T, p.Met256Ile) that was also predicted to be pathogenic. Localization of OPN3 protein by immunohistochemical analysis using mouse embryo revealed its specific expression in the tooth gems from bud to bell stages and their surrounding tissues. These results indicated that OPN3 was involved in non-syndromic oligodontia, which has made an anchoring point for clinical application including DNA diagnostics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
06 December 2021
A Correction to this paper has been published: https://doi.org/10.1038/s10038-021-00997-9
References
Schalk-van der Weide Y, Steen WH, Bosman F. Distribution of missing teeth and tooth morphology in patients with oligodontia. ASDC J Dent Child. 1992;59:133–40.
Ruf S, Klimas D, Honemann M, Jabir S. Genetic background of nonsyndromic oligodontia: a systematic review and meta-analysis. J Orofac Orthop. 2013;74:295–308.
Higashihori N, Takada JI, Katayanagi M, Takahashi Y, Moriyama K. Frequency of missing teeth and reduction of mesiodistal tooth width in Japanese patients with tooth agenesis. Prog Orthod. 2018;19:30.
Goya HA, Tanaka S, Maeda T, Akimoto Y. An orthopantomographic study of hypodontia in permanent teeth of Japanese pediatric patients. J Oral Sci. 2008;50:143–50.
Yin W, Bian Z. The gene network underlying hypodontia. J Dent Res. 2015;94:878–85.
Tatematsu T, Kimura M, Nakashima M, Machida J, Yamaguchi S, Shibata A, et al. An aberrant splice acceptor site due to a novel intronic nucleotide substitution in MSX1 gene is the cause of congenital tooth agenesis in a Japanese family. PLoS ONE 2015;10:e0128227.
Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140:2530–5.
Bergendal B, Klar J, Stecksén-Blicks C, Norderyd J, Dahl N. Isolated oligodontia associated with mutations in EDARADD, AXIN2, MSX1, and PAX9 genes. Am J Med Genet A. 2011;155a:1616–22.
Endo T, Ozoe R, Kubota M, Akiyama M, Shimooka S. A survey of hypodontia in Japanese orthodontic patients. Am J Orthod Dentofac Orthop. 2006;129:29–35.
Tabata MJ, Matsumura T, Liu JG, Wakisaka S, Kurisu K. Expression of cytokeratin 14 in ameloblast-lineage cells of the developing tooth of rat, both in vivo and in vitro. Arch Oral Biol. 1996;41:1019–27.
Blackshaw S, Snyder SH. Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19:3681–90.
Terakita A. The opsins. Genome Biol. 2005;6:213.
Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 2015;91:117–23.
Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45.
Barreto Ortiz S, Hori D, Nomura Y, Yun X, Jiang H, Yong H, et al. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am J Physiol Lung Cell Mol Physiol. 2018;314:L93–l106.
Kumbalasiri T, Provencio I. Melanopsin and other novel mammalian opsins. Exp Eye Res. 2005;81:368–75.
Buscone S, Mardaryev AN, Raafs B, Bikker JW, Sticht C, Gretz N, et al. A new path in defining light parameters for hair growth: discovery and modulation of photoreceptors in human hair follicle. Lasers Surg Med. 2017;49:705–18.
Ozdeslik RN, Olinski LE, Trieu MM, Oprian DD, Oancea E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc Natl Acad Sci USA. 2019;116:11508–17.
Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523:561–7.
Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502.
Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–7.
Latorraca NR, Venkatakrishnan AJ, Dror RO. GPCR dynamics: structures in motion. Chem Rev. 2017;117:139–55.
Choe HW, Park JH, Kim YJ, Ernst OP. Transmembrane signaling by GPCRs: insight from rhodopsin and opsin structures. Neuropharmacology. 2011;60:52–7.
Acknowledgements
We would like to thank all participants and their families for the involvement in this study. We also thank Ms. Makiko Matsuda, Makoto Sugiura, and Momoko Sakaguchi for their kind support and advice to the study. This work was supported in part by JSPS KAKENHI Grant Number 19K10376. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article. A supplemental materials to this article is available online.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Toshihiro Tanaka is Editor-in-Chief of Journal of Human Genetics. He was not involved in the peer-review or handling of the manuscript. The authors have no other competing interests to disclose.
Web Resources
The URLs for the data presented here are as follows: 1000 Genome Project, http://1000genome.org. dbSNP, https://www.ncbi.nlm.nih.gov/snp/. Human Genetic Variation Database, http://www.genome.med.kyoto-u.ac.jp. jMorp, https://jmorp.megabank.tohoku.ac.jp. gnomAD (ExAC), https://gnomad.broadinstitute.org. Mutation Taster, http://www.mutationtaster.org. National Heart, Lung, and Blood Institute Exome Sequencing Project, http://evs.gs.washington.edu/EVS. Online Mendelian Inheritance in Man, http://www.omim.org. PolyPhen-2, http://www.genetics.bwh.harvard.edu/pph2. PROVEAN, http://provean.jcvi.org/index.php. RefSeq, http://www.ncbi.nlm.nih.gov/RefSeq. SIFT, http://sift.bii.a-star.edu.sg
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Inagaki, Y., Ogawa, T., Tabata, M.J. et al. Identification of OPN3 as associated with non-syndromic oligodontia in a Japanese population. J Hum Genet 66, 769–775 (2021). https://doi.org/10.1038/s10038-021-00903-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-021-00903-3