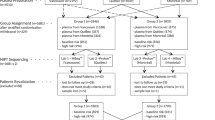
Abstract
Triploidy is a genetic aberration resulting from an extra haploid set of chromosomes of paternal (diandric) or maternal (digynic) origin. Diandric cases, opposite to digynic ones, may lead to gestational trophoblastic neoplasia (GTN) or generate maternal complications, therefore their identification is crucial, but reproducibility of traditionally used histopathological assessment is poor. The aim of the study was to analyse the usefulness of methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) with probes for two differentially methylated regions (DMR) at chromosome 11p.15.5 for identification of the parental origin of triploidy. 84 triploid DNA samples were tested with MS-MLPA: 34 paternal cases (40.5%) and 50 maternal ones (59.5%) according to the reference results of QF-PCR. Methylation ratio (MR) was calculated. Reference values proposed by the MRC-Holland for diploid samples (MR 0.8–1.2) were used. The values outside these ranges were used to diagnose parental origin of triploidy—paternal (MR > 1.2) or maternal (MR < 0.8). The effectiveness of MS-MLPA was 94.0%. The mean MR in paternal triploidy was 1.7 (SD–0.25; n = 34) compared with 0.56 in maternal triploidy (SD–0.12; n = 50). MR values in paternal and maternal triploidy did not overlap. In five samples (6.0%) parental origin of triploidy could not be accurately established by MS-MLPA, probably due to the maternal cell contamination (MCC). MS-MLPA can be used as a convenient method for distinguishing between paternal and maternal triploidy without the necessity for parental samples testing. It enables adequate selection of the paternal triploid cases for follow up in order to exclude post-molar GTN.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jacobs PA, Szulman AE, Funkhouser J, Matsuura JS, Wilson CC. Human triploidy: relationship between parental origin of the additional haploid complement and development of partial hydatidiform mole. Ann Hum Genet. 1982;46:223–31.
McFadden DE, Langlois S. Parental and meiotic origin of triploidy in the embryonic and fetal periods. Clin Genet. 2000;58:192–200.
McFadden DE, Kwong LC, Yam IY, Langlois S. Parental origin of triploidy in human fetuses: evidence for genomic imprinting. Hum Genet. 1993;92:465–9.
Seckl MJ, Fisher RA, Salerno G, Rees H, Paradinas FJ, Foskett M, et al. Choriocarcinoma and partial hydatidiform moles. Lancet. 2000;356:36–9.
Rijhsinghani A, Yankowitz J, Strauss RA, Kuller JA, Patil S, Williamson RA. Risk of preeclampsia in second-trimester triploid pregnancies. Obstet Gynecol. 1997;90:884–8.
Massalska D, Bijok J, Kucińska-Chahwan A, Zimowski JG, Ozdarska K, Raniszewska A, et al. Maternal complications in molecularly confirmed diandric and digynic triploid pregnancies: single institution experience and literature review. Arch Gynecol Obstet. 2020;301:1139–45.
McFadden DE, Kalousek DK. Two different phenotypes of fetuses with chromosomal triploidy: correlation with parental origin of the extra haploid set. Am J Med Genet. 1991;38:535–8.
Zaragoza MV, Surti U, Redine RW, Millie E, Chakravarti A, Hassold TJ. Parental origin and phenotype of triploidy in spontaneous abortions: predominance of diandry and association with the partial hydatidiform mole. Am J Hum Genet. 2000;66:1807–20.
Joergensen MW, Niemann I, Rasmussen AA, Hindkjaer J, Agerholm I, Bolund L, et al. Triploid pregnancies: genetic and clinical features of 158 cases. Am J Obstet Gynecol. 2014;211:370.e1–370.e19.
Fukunaga M, Katabuchi H, Nagasaka T, Mikami Y, Minamiguchi S, Lage JM. Interobserver and intraobserver variability in the diagnosis of hydatidiform mole. Am J Surg Pathol. 2005;29:942–7.
Murphy KM, McConnell TG, Hafez MJ, Vang R, Ronnett BM. Molecular genotyping of hydatidiform moles: analytic validation of a multiplex short tandem repeat assay. J Mol Diagn. 2009;11:598–605.
Bourque DK, Penaherrera MS, Yuen RK, Van Allen MI, McFadden DE, Robinson WP. The utility of quantitative methylation assays at imprinted genes for the diagnosis of fetal and placental disorders. Clin Genet. 2011;79:169–75.
Joergensen MW, Rasmussen AA, Niemann I, Hindjaer J, Agerholm I, Bolund L, et al. Methylation-specific multiplex ligation-dependent probe amplification: utility for prenatal diagnosis of parental origin in human triploidy. Prenat Diagn. 2013;33:1131–6.
Choufani S, Shuman C, Weksberg R. Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:343–54.
Massalska D, Bijok J, Zimowski JG, Jóźwiak A, Jakiel G, Roszkowski T. Multiplex ligation-dependent probe amplification (MLPA)—new possibilities of prenatal diagnosis. Ginekol Pol. 2013;84:461–4.
Nygren AO, Ameziane N, Duarte HM, Vijzelaar RN, Waisfisz Q, Hess CJ. et al.Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences.Nucleic Acids Res.2005;33:e128.
Lathi RB, Gustin SL, Keller J, Maisenbacher MK, Sigurjonsson S, Tao R, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178–82.
Zimowski JG, Massalska D, Pawelec M, Bijok J, Michałowska A, Roszkowski T. First-trimester spontaneous pregnancy loss—molecular analysis using multiplex ligation-dependent probe amplification. Clin Genet. 2016;89:620–4.
Coyle C, Short D, Jackson L, Sebire NJ, Kaur B, Harvey R, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol. 2018;48:254–7.
Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM, et al. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131:3727–35.
Lewis A, Mitsuya K, Umlauf D, Smith P, Dean W, Walter J, et al. Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat Genet. 2004;36:1291–5.
Monk D, Arnaud P, Apostolidou S, Hills FA, Kelsey G, Stanier P, et al. Limited evolutionary conservation of imprinting in the human placenta. Proc Natl Acad Sci USA. 2006;103:6623–8.
Funding
The study was partly funded by Centre of Postgraduate Medical Education in Warsaw (Project No. 501-1-21-27-18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Massalska, D., Ozdarska, K., Bijok, J. et al. Usefulness of methylation-specific multiplex ligation-dependent probe amplification for identification of parental origin of triploidy. J Hum Genet 65, 889–894 (2020). https://doi.org/10.1038/s10038-020-0784-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-020-0784-0
This article is cited by
-
Distribution of diandric and digynic triploidy depending on gestational age
Journal of Assisted Reproduction and Genetics (2021)