Abstract
The data collected by nation-wide study of noninvasive prenatal genetic testing (NIPT) for trisomy 21 from 21,610 pregnant women with advanced maternal age in Japan were reported. Among 188 NIPT-positive cases, 180 cases were true positive. The incidence of aneuploidy according to maternal age was estimated using a state-space model. Although, the frequency of trisomy increased exponentially with maternal age as previously reported, the maternal age-specific risk for trisomy 21 that was based on the clinical performance of NIPT was lower than the predicted risk in previous Western cohorts based on the data from invasive prenatal testing (Bayesian two-sided tail-area probability P = 0.0156). The empirical positive predictive value (PPV) of NIPT is likely to turn out higher than that of the theoretical PPV calculated from the sensitivity/specificity of the test and the incidence of trisomy 21 from this study.
Similar content being viewed by others
Introduction
Information regarding the live birth incidence of trisomy is indispensable in prenatal genetic counseling. The incidence at different stages of pregnancy is also useful for identifying necessary prenatal genetic tests [1]. The sensitivity and specificity are used to assess the efficacy of each screening test and the positive predictive value (PPV) and negative predictive value (NPV) are of great concern in clinical testing because they depend on prior probability, or maternal age-specific and gestational age-specific risks of trisomy. The maternal age and gestational age-specific risks have been previously reported [1,2,3], and are calculated using the data from chorionic villus sampling (CVS) (10–14 weeks), amniocentesis (15–20 weeks), and live birth (term) cases.
NIPT is a noninvasive and highly reliable screening test. Unlike invasive procedures, NIPT carries no risk of miscarriage or harm to the unborn child, and the characteristics of NIPT clients at advanced maternal age are similar to those of the natural population.
In Japan, NIPT is not a standard medical test, is permitted only as part of clinical research, and all the collected data are always secured and controlled.
The objective of the present study is to empirically estimate the maternal age-specific risk for trisomy 21 using the data collected by nation-wide NIPT research and maternal age-specific NIPT-PPV and NIPT-NPV, which is based on clinical NIPT data in Japan.
Method
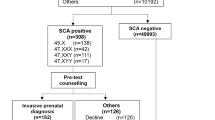
This was a multicenter prospective cohort study conducted in Japan with the approval of the institutional review board of ethics at each respective institution. The data were collected from a total of 21,610 pregnant women undergoing NIPT (massively parallel sequencing: MaterniT21 Plus® and GeneTech NIPT) with advanced maternal age (age: 38.48 ± 0.02, gestational weeks: 13.36 ± 0.01) between April 2013 and December 2015 at 38 medical institutions in Japan. To avoid any biased feedback, the data from the patients who had undergone ultrasound screening before NIPT were excluded.
The estimated risk of trisomy 21 for our data set was compared with that of previously reported data from Western countries [1, 2, 4–7].
The incidence of aneuploidy according to maternal age was estimated using a state-space model [8,9,10]. The incidence of aneuploidy and observed aneuploidy were modeled according to the following two formulae (where Eq. 1 denotes state process and Eq. 2 denotes observed process):
where t was maternal age, \(p_t \in (0,1)\) was incidence to be estimated, \(w_t \in \{ 0,1,2 \ldots \}\) was the observation count of aneuploidy, and ϵt was Gaussian noise. The incidence of aneuploidy generally monotonically increases according to maternal age [1, 2, 4, 6]. Monotonic increase was assumed to occur according to the formula:
Besides estimating the incidence of aneuploidy in our data set, we estimated the incidence in other data sets from Australia [2] and Japan [3] as a reference. Estimation was performed using R 3.4.0 (https://cran.r-project.org/) and rstan 2.15.1 (http://mc-stan.org).
PPV and NPV were calculated exclusively based on the data from the patients whose birth outcomes were confirmed.
In this study, “theoretical PPV” (\({\rm{PPV}}_t\)) and “empirical PPV” (\({\rm{PPV}}_e\)) were defined as following:
where p was incidence of trisomy, Sn was sensitivity, Sp was specificity, wtrue was the number of the true trisomy confirmed by diagnostic test, and wNIPT was the number of positive cases in NIPT data set.
The theoretical PPV and NPV for trisomy 21 were calculated based on a sensitivity of 99.1% and specificity of 99.9% that were reported by Palomaki et al. in the validation study of MaterniT21 Plus® [11].
Results
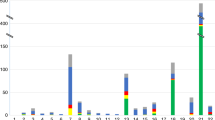
The number of NIPT-positive cases for trisomy 21 was 188, of which 180 cases were confirmed by invasive testing or karyotyping of POC (PPV 95.7%) (Table 1, Fig. 1). The negative predictive value was 100% because there were no false-negatives identified in this cohort (Fig. 1). Among 21,381 NIPT-negative cases, the cases of which outcomes were confirmed were 16,585. There was no false-negative case in 16,585 cases. In another paper of our study group [12], there were two false-negative cases (one trisomy 21 and one trisomy 18) in 13,491 cases. These cases were not included in this study because the indication of these cases was abnormal findings of ultrasound. The frequency of trisomy increased exponentially with maternal age, as previously reported [1,2,3,4,5,6,7] (Table 1, Fig. 2a). However, the maternal age-specific risk for trisomy 21 during the CVS period (10–14 weeks) that was based on the clinical performance of NIPT was lower than the predicted risk [1] in Western cohorts (Bayesian two-sided tail-area probability p = 0.0156) (Fig. 2a), while the risk for trisomy 18 and 13 were approximately the same (data not shown).
NIPT results and outcomes for detection of trisomy 21 in 21,610 women. ‘Confirmed outcomes’ represents clinical confirmation of a healthy baby, or actual karyotyping. IUFD intra uterine fetal death without karyotyping, AA artificial abortion without karyotyping, HB healthy baby, AC karyotyping with amniocentesis, POC karyotyping with product of conception after IUFD
We then calculated the maternal age-specific PPV curve from the maternal age-specific risk for trisomy 21, the detection rates, and the test specificities (Fig. 3). The risk according to the empirical data was higher than that according to the theoretical data, especially among younger age groups.
Maternal age-specific positive predictive value (PPV) of trisomy 21 at 14 weeks of gestation. The color gradation represents the variation in NIPT specificity. The dark blue line located in the bottom of the graph is the PPV curve, which was calculated from the specificity reported by Palomaki et al. (99.90%) [11]. The pink line is the PPV curve, which was calculated based on Snijders et al. [1]. The black line is the regression curve of the outlier model from the empirical data obtained in the present study. The empirical PPV was much higher than the theoretical PPV, especially among younger age groups. The theoretical PPV increased with increasing specificity
Discussion
The first aim of the present study was to empirically estimate the maternal age-specific risk for trisomy 21 using NIPT data in Japan. For evaluating the obtained risk for trisomy 21, we compared our data with previous studies. The maternal age and gestational age-specific incidence of trisomy as reported by Snijders et al. [1] is often cited in established textbooks [13]. However, our data of maternal age-specific risk for trisomy 21 was much lower than that of Snijders et al. [1]. We re-evaluated the available data by comparing other independent studies. Although the maternal age-specific risk of trisomy reported by Kratzer [5] was similar to that reported by Snijders et al. [1], Halliday [2] confirmed the results of the present study. In comparing studies based on amniocentesis period (Fig. 2b), and term (Fig. 2c) with other studies, large variation was observed among studies, especially in mothers older than 41 years, while the maternal age-specific risks reported by Snijders was much higher. The risk of trisomy 21 might be lower in Japanese people, as the Japanese risk seems to be the lowest based on CVS (our NIPT data) and amniocentesis periods [3]. Other studies [2,3,4,5,6,7], have also indicated that the incidence of trisomy 21 at each gestational period is lower than that reported by Snijders et al. [1]. Our data might therefore represent a more accurate depiction of the trisomy risk than that of Snijders [1]. The results of the present study could be used to evaluate the significance of NIPT data as well as that of other screening tests (maternal serum markers, combined testing, etc.). In Japan, the artificial reproductive technology is prevalent, especially among advanced maternal age. Limitations of this study include that the age of oocyte retrieval was not considered in the analysis.
Empirical NIPT/PPV is likely to turn out higher than the theoretical PPV extracted from the lower trisomy 21 incidence, which our study showed, and this is especially true for younger generation. Although the etiology remains unknown, we can postulate a possible explanation. For the calculation of theoretical PPV, we used the sensitivity (99.1%) and specificity (99.9%) reported by Palomaki et al. [11]. However, the actual specificity might be much higher now than it was 5 years ago. When we assume the specificity is 99.98%, the theoretical PPV curve rises and is similar to the curve based on the empirical data (Fig. 3). At the least, this is an area that can use further research; A larger sample size in NIPT positive will allow empirical PPVs to be more accurate, but it is safe to say that what is found in this study is rather significant.
In Japan, NIPT is not a standard medical test, but a clinical research performed for patients in higher risk groups. NIPT is permitted only for high-risk pregnant women, including those with advanced maternal age, because the utility of NIPT for low-risk pregnant women had not been established when the Japanese guidelines on new prenatal genetic testing methods using maternal blood were released [12]. As a result, young women may not receive NIPT even if they request it. Recently, in some non-approved clinics, some low-risk pregnant women have received NIPT without proper genetic counseling, resulting in serious problems for patients who received positive results. These patients do not receive the appropriate genetic counseling, and suffer from persistent anxiety, often visiting our hospitals and demanding proper genetic counseling and care. Although some evidence of the efficiency of NIPT in low-to-middle-risk women [14, 15] has been reported, since the release of the guidelines, the guidelines have not been updated. The present study provides further evidence to support the utility of NIPT for low-risk pregnant women, and prompt the revision of the guidelines.
Change history
01 August 2018
Since the advance online publication of this article, the authors of the above paper have noticed errors in the list of authors and affiliations. The article with correct author information now appears in this issue.
References
Snijders RJ, Sebire NJ, Nicolaides KH. Maternal age and gestational age-specific risk for chromosomal defects. Fetal Diagn Ther. 1995;10:356–67.
Halliday JL, Watson LF, Lumley J, et al. New estimates of Down syndrome risks at chorionic villus sampling, amniocentesis, and livebirth in women of advanced maternal age from a uniquely defined population. Prenat Diagn. 1995;15:455–65.
Yaegashi N, Senoo M, Uehara S, et al. Age-specific incidences of chromosome abnormalities at the second trimester amniocentesis for Japanese mothers aged 35 and older: collaborative study of 5484 cases. J Hum Genet. 1998;43:85–90.
Cuckle HS, Wald NJ, Thompson SG. Estimating a woman’s risk of having a pregnancy associated with Down’s syndrome using her age and serum alpha-fetoprotein level. Br J Obstet Gynaecol. 1987;94:387–402.
Kratzer PG, Golbus MS, Schonberg SA, et al. Cytogenetic evidence for enhanced selective miscarriage of trisomy 21 pregnancies with advancing maternal age. Am J Med Genet. 1992;44:657–63.
Hecht CA, Hook EB. Rates of Down syndrome at livebirth by one-year maternal age intervals in studies with apparent close to complete ascertainment in populations of European origin: a proposed revised rate schedule for use in genetic and prenatal screening. Am J Med Genet. 1996;62:376–85.
Morris JK, Wald NJ, Mutton DE, Alberman E. Comparison of models of maternal age-specific risk for Down syndrome live births. Prenat Diagn. 2003;23:252–8.
Ricker WE. Stock and recruitment. J Fish Board Can. 1954;11:559–623.
Commandeur JJF, Koopman SJ. An introduction to state space time series analysis (practical econometrics). Oxford University Press Inc.: New York, 2007. p. 135–54
Nadeem K, Moore JE, Zhang Y, Chipman H. Integrating population dynamics models and distance sampling data: a spatial hierarchical state-space approach. Ecology. 2016;97:1735–45.
Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med. 2012;14:296–305.
Samura O, Sekizawa A, Suzumori N, et al. Current status of non-invasive prenatal testing in Japan. J Obstet Gynaecol Res. 2017;43:1245–55.
Gardner RJM, Sutherland GR, Shaffer LG. Chromosome abnormalities and genetic counseling. 4th ed. New York: Oxford University Press, Inc.; 2012. p. 405–7.
Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799–808.
Fairbrother G, Johnson S, Musci TJ, Song K. Clinical experience of noninvasive prenatal testing with cell-free DNA for fetal trisomies 21, 18, and 13, in a general screening population. Prenat Diagn. 2013;33:580–3.
Acknowledgements
We thank all the members of the Japan NIPT consortium, and all clinical geneticists and genetic counselors for their thoughtful cooperation on this project. This study was supported by the Grant of National Center for Child Health and Development 24-3, Japan (to AS and HS).
Funding
This work was supported by the Grant of National Center for Child Health and Development 24-3, Japan.
Study collaborators of Japan NIPT Consortium:
Takashi Kaji (The University of Tokushima Faculty of Medicine), Masanobu Ogawa (National Hospital Organization Kyushu Medical Center), Keiichi Matsubara (Ehime University School of Medicine), Haruka Hamanoue (Yokohama City University Graduate School of Medicine), Akimune Fukushima (Iwate Medical University School of Medicine), Masayuki Endo (Osaka University), Kazufumi Haino (Niigata University Medical and Dental Hospital), Hideaki Masuzaki (Nagasaki University Graduate School of Biomedical Sciences), Masaki Ogawa (Tokyo Women’s Medical University Hospital), Shinya Tairaku (Kobe University Graduate School of Medicine), Masato Mizuuchi (Sapporo Medical University School of Medicine), Yoko Okamoto (Osaka Medical Center and Research Institute for Maternal and Child Health), Yukie Kawano (Oita University), Hisashi Masuyama (Okayama University Graduate School of Medicine), Hisao Osada (Chiba University Graduate School of Medicine), Taihei Tsunemi (Nara Medical University), Kazuhisa Maeda (Shikoku Medical Center for Children and Adults), Yasuyo Kasai (Japanese Red Cross Medical Center), Reiko Neki (National Cerebral and Cardiovascular Center), Yukiko Katagiri (Toho University Omori Medical Center), Shunichiro Izumi (Tokai University School of Medicine), Setsuko Nakayama (Aiiku Hospital), Yuko Yokohama (Asahikawa Medical University), Masaya Hirose (Hyogo Prefectural Amagasaki General Medical Center), Kousuke Kawakami (National Hospital Organization Kokura Medical Center), Kiyotake Ichizuka (Showa University Northern Yokohama Hospital), Masakatsu Sase (Yamaguchi Grand Medical Center), Satoru Sakatsume (Dokkyo Medical University Koshigaya Hospital), Tomohiko Tsuruta (Kansai Rosai Hospital)
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Study Collaborators of Japan NIPT Consortium are described separately.
Rights and permissions
About this article
Cite this article
Yamada, T., Sekizawa, A., Fujii, Y. et al. Maternal age-specific risk for trisomy 21 based on the clinical performance of NIPT and empirically derived NIPT age-specific positive and negative predictive values in Japan. J Hum Genet 63, 1035–1040 (2018). https://doi.org/10.1038/s10038-018-0453-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-018-0453-8
This article is cited by
-
Efficiency of non-invasive prenatal screening in pregnant women at advanced maternal age
BMC Pregnancy and Childbirth (2021)
-
Evidence of compliance with and effectiveness of guidelines for noninvasive prenatal testing in China: a retrospective study of 189,809 cases
Science China Life Sciences (2020)
-
Next-generation sequencing: a follow-up of 36,913 singleton pregnancies with noninvasive prenatal testing in central China
Journal of Assisted Reproduction and Genetics (2020)