Abstract
Pulmonary disease in cystic fibrosis (CF) is characterized by a chronic neutrophil-dominated inflammation of lung tissue. Inasmuch as some amino acids (AA) play a pivotal role in various aspects of neutrophil metabolism, the aim of this study was to investigate a possible alteration of neutrophil AA metabolism and to evaluate its relation (if any) with the genotype. We performed plasma and neutrophil AA analysis in 26 CF patients with known genotype, 10 patients with non-CF bronchiectasis, and 20 normal subjects. The CF group showed a significant decrease of free intracellular neutrophil glutamine (Gln) content compared with controls and the non-CF bronchiectasis group. In the latter group, levels of neutrophil Gln were significantly lower compared with the controls. Amino acid plasma concentration in non-CF bronchiectasis showed a decrease of Gln and taurine compared with controls. Neutrophil Gln content showed values significantly lower in CF patients with severe mutations (class I, II, and III mutations) compared with mild mutations (class IV and V mutations). Results of our study add further evidence for intrinsic neutrophil alterations that could play an important role in the pathogenesis of chronic pulmonary disease in CF patients.
Similar content being viewed by others
Main
One of the clinical hallmarks of CF is chronic inflammatory lung disease dominated by polymorphonuclear neutrophil influx in the airways (1). Neutrophils release massive amounts of active proteases, including elastase, which is considered to be the major mediator of the observed lung damage. This inappropriate neutrophil emigration has been considered to result from a defective regulation of the inflammatory response in the lung and is mainly attributed to local factors like abnormal secretion of chemoattractants by respiratory epithelial cells (2). More recent studies showed that neutrophils from CF patients have intrinsic functional alterations that modify their response to inflammatory stimuli, suggesting the presence of more complex systemic interactions in the pathogenesis of lung disease (3–7). Furthermore, in the last decade evidence is accumulating that the basic genetic defect in CFTR may contribute not only to an increased propensity to pulmonary infection (8,9) but also to a defective regulation of the inflammatory response (10,11).
It is well known that some AA play a pivotal role in various aspects of neutrophil metabolism, including protein synthesis and energy production. Taurine, a nonproteic sulfur b-AA, being the major intracellular free AA, is known to be an antioxidant and membrane stabilizing agent (12). This AA is particularly abundant in neutrophils, where it protects these cells from damage induced by chlorinated oxidants during oxidative burst (13). Arginine and citrulline are, respectively, substrate and end-product of nitric oxide biosynthesis (14), a pathway involved in neutrophil bacterial killing as well as in immune response regulation (14,15). Gln is involved in several key metabolic processes in neutrophils, as a precursor of nucleotides for RNA and DNA synthesis and substrate for energy production and NADPH synthesis through the “glutaminolysis” pathway (16). These metabolic functions are essential to sustain a number of different cellular processes, including motility, respiratory burst, and secretion of cytoplasmic proteolytic enzymes and immunomodulatory compounds involved in the initiation of phagocytosis and bacterial killing (17).
The aim of this study was to investigate a possible alteration of AA neutrophil metabolism in children affected by CF and to evaluate its relation (if any) with the genotype. Thus, we determined plasma AA concentration and free intracellular AA content in circulating neutrophils from CF patients with mutations associated with complete failure or residual CFTR activity. As comparative groups, we included normal controls and subjects affected by non-CF bronchiectasis.
METHODS
Subjects.
Twenty-six patients with CF confirmed by genotyping and positive sweat test (15 male, 11 female; age, 4–18 y; median, 11.5 y) were enrolled in the study when attending an outpatient clinic for routine evaluation of disease in the Department of Pediatrics, University of Rome. Blood samples for plasma and neutrophil AA analysis were collected at the same time. At the time of sampling, no patient had evidence of acute infection. Exclusion criteria included also the presence of liver disease, alterations of endocrine pancreas function, use of systemic steroids, or nonsteroidal antiinflammatory medications during the last 2 mo or recent use of antibiotics. Eight patients (2 male, 6 female; age, 8–18 y; median, 10.7 y) showed evidence of chronic PA colonization obtained by repeated positive sputum cultures. Spirometric assessment of pulmonary function was obtained in 18 patients aged older than 8 y. Results were expressed as FEV1% and indicated a full range of disease severity (median, 88%; range, 49–141%). Rx-thorax assessment of pulmonary disease was accomplished by a systematic evaluation score (median, 10.5; range, 3–30) (18). Clinical assessment of illness severity was performed by the Shwachman-Kulczycki score (mean ± SD, 82.58 ± 10.3) (19). Nutritional status was evaluated using the Moore index (mean ± SD, 100.6 ± 11.2) (20). All these clinical data were recorded within 2 mo before or after sampling. Results are shown in Table 1. Fifteen CF patients (8 male, 7 female; age, median 10.8 y; range, 4–18 y) were homozygous or compound heterozygous for mutations known to produce a complete failure of CFTR activity (class I, II, and III mutations), whereas the remaining 11 patients (7 male, 4 female; age, median 12.1 y; range, 4–18 y) had mutations associated with a residual CFTR activity (class IV and V mutations). The reference normal control group consisted of 20 healthy subjects (11 male, 9 female; age, 4–18 y; median, 11.3 y) showing no alteration of systemic inflammatory component who underwent routine hematological assessments. Disease controls comprised 10 patients with non-CF bronchiectasis (5 male, 5 female; age, 6–18 y; median, 12.1 y) without signs of acute exacerbation.
Informed consent was obtained from all parents and from all patients older than 11 y. The study was approved by the Ethics Committee of Department of Pediatrics, University “La Sapienza,” Rome.
Neutrophil isolation procedure.
Neutrophils were isolated as previously described (21) from heparinized blood, by dextran sedimentation, Ficoll-Hypaque gradient, centrifugation, and subsequent removal of contaminating erythrocytes with hypotonic lysis. The resulting cell preparation was resuspended in calcium- and magnesium-free PBS with glucose (125 mM NaCl, 8 mM Na2HPO4, 2 mM NaH2PO4·H2O, 5 mM KCl, 5 mM glucose; pH 7.4) and stored on ice until analysis. Cells were counted and viability determined by trypan blue dye exclusion test. The purity of the neutrophil suspension was >95%.
Plasma AA preparation.
Venous K2 EDTA blood (3 mL) was collected after overnight fasting and immediately placed on ice. Plasma was obtained by centrifuging blood at 850 g for 1 h at 4°C. Sample preparation was accomplished according to the Pico-Tag method (Waters, Milford, MA) for physiologic AA (22). One milliliter of plasma was diluted 1:1 with 0.1 M HCL containing 0.13 g/L norleucine (Sigma Chemical Co.-Aldrich, Milan, Italy) as internal standard. Aliquots of 200 mL were promptly deproteinized by ultrafiltration using Ultrafree MC microcentrifuge devices (Millipore, Bedford, MA) with a 10,000 molecular weight cutoff limit. Aliquots of 20 mL of the ultrafiltrate were desiccated under vacuum and derivatized with 20 mL of methanol-water-triethylamine-phenylisothiocyanate solution (7:1:1:1, respectively). Excess solvents and reagents were eliminated under vacuum and samples were stored at –20°C until chromatographic analysis.
Neutrophil AA extraction.
Neutrophils were suspended in 200 mL of water and ultrasonicated in a Sonicator Cell Disruptor applied in a pulse basis (23). To avoid heating, samples were kept in ice-water bath during sonication. Neutrophil lysates were then centrifuged at 1000 rpm for 20 min at 4°C to sediment cellular debris. Aliquots of 200 mL were promptly deproteinized by ultrafiltration as previously described for plasma.
AA analysis.
The phenylthiocarbamyl derivatives of plasma and neutrophil AA were assayed by reverse-phase HPLC using the Pico-Tag method (22). The column was an application-specified C18 Pico-Tag column (30 cm × 3.9 mm, Waters). Derivatized AA were detected by measuring the absorbance at 254 nm. The chromatographic system was a liquid chromatograph detector (Waters) consisting of two 510 HPLC pumps for high-pressure gradient, a temperature control module (set at 46°C), a 484 absorbance detector, a Rheodine 20 mL injector, and the Millennium Chromatography data acquisition and management software (Waters).
Statistical analysis.
Data are reported as mean ± SD unless otherwise stated. Comparison between groups (CF, non-CF bronchiectasis, and control subjects) were made using a two-tailed t test. Given that the data were approximately log-normally distributed (Fig. 1), they were log-transformed before statistical analysis. Correlations were performed by linear regression analysis. Statistical significance was set at p < 0.05.
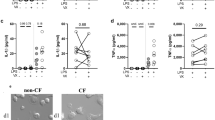
Neutrophil glutamine content in CF patients with mutations associated with a complete failure of CFTR activity (CF severe, n = 15) and CF patients with mutations associated with a residual CFTR activity (CF mild, n = 11), non-CF bronchiectasis (n = 10), and controls (n = 20). Neutrophil glutamine content in “CF severe” showed values significantly reduced compared with the “CF mild” genotype (p < 0.01) and the non-CF bronchiectasis and controls (p < 0.001).
RESULTS
The results of AA content in neutrophils, expressed as micromoles × 10–7 are reported in Table 2. The CF group showed a significant decrease of free intracellular neutrophil Gln content compared with controls (mean, 4.31 ± 2.72 versus 10.6 ± 3.48; p < 0.001) and the non-CF bronchiectasis group (mean, 4.31 ± 2.72 versus 7.67 ± 4.32; p < 0.01). In the latter group, levels of neutrophil Gln were significantly lower in respect to the controls (p < 0.03). Total intracellular AA were significantly increased in the CF group compared with controls (450.9 ± 112.2 versus 324.3 ± 90.42; p < 0.001).
AA plasma concentration (μmol/L) in non-CF bronchiectasis showed a decrease of Gln (p < 0.01) and taurine (p < 0.01) compared with controls, whereas in the CF group no significant differences were found (Table 3).
In the CF group, a weak but significant correlation between plasma Gln concentration and neutrophil Gln content was found (r2 = 0.47; p < 0.05). Plasma concentration and neutrophil content of Gln, taurine, arginine, and citrulline did not show any significant correlation with age, inflammatory parameters (CRP, white blood cell count), indexes of pulmonary and disease severity (FEV1%, Rx-thorax score, and Shwachman-Kulczycki score, respectively) and nutritional status (Moore index). No significant differences in clinical and biochemical parameters were found between CF patients with and without PA colonization. No significant correlation was found between plasma concentration and neutrophil Gln content in non-CF bronchiectasis and controls.
To investigate the role of genotype in determining the biochemical alterations of neutrophils in CF, we considered two subgroups sharing severe and mild mutations, respectively, as reported in “Methods.” Neutrophil Gln content showed values significantly lower in the former group (2.95 ± 1.40 versus 6.16 ± 3.03; p < 0.01) (Fig. 1).
Finally, we found in the CF group increased neutrophil content of glycine, histidine, threonine, isoleucine, and valine in respect to the controls, but these results were not considered as primary outcomes in our study.
DISCUSSION
The main result of our study was a marked decrease of Gln content in circulating neutrophils and normal plasma values in CF patients, whereas non-CF bronchiectasis showed a mild intracellular Gln depletion with decreased plasma concentration. Moreover, patients with “severe” CFTR mutations showed values of neutrophil Gln content significantly lower in respect to those with “mild” mutations, suggesting a role of genetic component in neutrophil metabolism.
Glutamine is normally considered to be a nonessential AA. However, studies have provided evidence that it may become “conditionally essential” during inflammatory condition and injury. In fact, studies performed in “critically ill patients” such as after burns, trauma, surgery, and sepsis showed a decrease of plasma, muscle, and neutrophil Gln content ascribed to elevated consumption in immunologic cells and other tissues resulting in a demand for Gln that outstrips supply (24–29). Furthermore, a recent study demonstrated a depletion of plasma and mucosal intestinal Gln correlated with the presence of chronic inflammatory stress (30). Our disease groups were both affected by a chronic inflammatory disease and were evaluated during a stable period. Nevertheless, we observed significant differences between them in respect to the plasma and intracellular Gln level. Non-CF bronchiectasis showed alterations similar to those previously described in inflammatory conditions, whereas the CF group exhibited a different pattern of alterations. The presence of normal Gln plasma values in CF patients suggest that a systemic Gln deficiency does not occur in these patients, indicating an intrinsic mechanism of neutrophil Gln depletion. This finding indicates that the presence of chronic inflammatory response is per se unable to explain the alteration of neutrophil Gln metabolism found in CF patients, suggesting that it could be directly correlated to the CFTR mutation. Interestingly, we found a significant difference in intracellular Gln content between the CF subgroups with severe or mild genotype, which supports the hypothesis of a direct correlation with the genetic background.
Evidence of the CFTR gene expression in cells of nonepithelial origin (including neutrophils) has been demonstrated in the past (31). More recently, the hypothesis that neutrophils of CF patients could have functional alterations directly correlated with CFTR mutation was proposed by Witko-Sarsot et al. (32). These authors found an increased ability to generate myeloperoxidase-derived oxidants from isolated neutrophils in heterozygous parents of CF patients, providing strong evidence for a genetic component to altered neutrophil function. It has been shown that circulating neutrophils have intrinsic alterations, likely due to CFTR mutation, implicating their primary involvement in the defective regulation of systemic inflammatory response in CF (4,5,33). Data reported here provide additional support for the presence of intrinsic alterations of neutrophil metabolism related to the genetic component that could represent a relevant factor explaining the changes of neutrophil behavior previously described. Recent studies have shown that Gln appears to exert a regulatory influence on inflammatory processes by neutrophils responding to inflammatory and infectious stimuli. In fact, Gln supplementation in vitro enhances both phagocytosis and ROS production in isolated neutrophils (34) and, in vivo, suppresses IL-8 production by neutrophils (35). Inasmuch as an increased IL-8 production (33) and oxidative burst alterations (36) have been reported in circulating neutrophils from CF patients, the Gln intracellular depletion found in our study may explain these alterations. However, the exact role of neutrophil Gln depletion in the pathogenesis of CF remains to be elucidated. We found no significant correlation between neutrophil Gln content and the main clinical parameters of pulmonary function and disease severity. Furthermore, these parameters did not show any significant difference between CF groups with severe or mild genotype. Nevertheless, on the basis of our results we cannot assert whether the neutrophil biochemical alterations observed are a cause rather than an effect of CF disease. Longitudinal clinical trials performed on a larger population and including phases of superimposed acute illness are needed.
In conclusion, we demonstrated that neutrophils of CF patients present biochemical alterations before migrating to the site of infection, and these alterations seem to be associated with CFTR mutation. Results of our study add further evidence for intrinsic neutrophil alterations that could play an important role in the pathogenesis of chronic pulmonary disease in CF patients.
Abbreviations
- AA:
-
amino acids
- CF:
-
cystic fibrosis
- CFTR:
-
cystic fibrosis transmembrane conductance regulator
- FEV1%:
-
percentage of predicted forced expiratory volume in 1 s
- Gln:
-
glutamine
- PA:
-
Pseudomonas aeruginosa
References
Ratjen F, Doring G 2003 Cystic fibrosis. Lancet 361: 681–689
Chmiel JF, Berger M, Konstan MW 2002 The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol 23: 5–27
Koller DY, Urbanek R, Gotz M 1995 Increased degranulation of eosinophil and neutrophil granulocytes in cystic fibrosis. Am J Respir Crit Care Med 152: 629–633
Russell KJ, McRedmond J, Mukherji N, Costello C, Keatings V, Linnane S, Henry M, Fitzgerald MX, O'Connor CM 1998 Neutrophil adhesion molecule surface expression and responsiveness in cystic fibrosis. Am J Respir Crit Care Med 157: 756–761
Taggart C, Coakley RJ, Greally P, Canny G, O' Neill SJ, McElvaney NG 2000 Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-alpha and interleukin-8. Am J Physiol Lung Cell Mol Physiol 278: L33–L41
Sagel SD, Accurso FJ 2002 Monitoring inflammation in CF. Clin Rev Allergy Immunol 23: 41–57
Coakley RJ, Taggart C, Canny G, Greally P, O' Neill SJ, McElvaney NG 2000 Altered intracellular pH regulation in neutrophils from patients with cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 279: L66–L74
Smith JJ, Travis SM, Greenberg EP, Welsh MJ 1996 Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85: 229–236
Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM 1997 Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88: 553–560
Moss R 1996 Pathways to inflammation in cystic fibrosis. Pediatr Pulmonol Suppl 13: 158–160
Berger M, Bonfield T, Konstan MW 1996 Cytokines in the lung in CF. Pediatr Pulmonol Suppl 13: 185–186
Stapleton PP, O'Flaherty L, Redmond HP, Bouchier-Hayes DJ 1998 Host defense—a role for the amino acid taurine?. J Parenter Enteral Nutr 22: 42–48
Learn DB, Fried VA, Thomas EL 1990 Taurine and hypotaurine content of human leukocytes. J Leukoc Biol 48: 174–182
Moncada S, Higgs A 1993 The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012
Norris KA, Schrimpf JE, Flynn JL, Morris SM Jr 1995 Enhancement of macrophage microbicidal activity: supplemental arginine and citrulline augment nitric oxide production in murine peritoneal macrophages and promote intracellular killing of Trypanosoma cruzi. Infect Immun 63: 2793–2796.
Curi R, Newsholme P, Pithon-Curi TC, Pires-de-Melo M, Garcia C, Homem-de-Bittencourt PI Jr, Guimaraes AR 1999 Metabolic fate of glutamine in lymphocytes, macrophages and neutrophils. Braz J Med Biol Res 32: 15–21
Curi TC, De Melo MP, De Azevedo RB, Zorn TM, Curi R 1997 Glutamine utilization by rat neutrophils: presence of phosphate-dependent glutaminase. Am J Physiol C1124–C1129
Chrispin AR, Norman AP 1974 The systematic evaluation of the chest radiograph in cystic fibrosis. Pediatr Radiol 2: 101–105
Shwachman H, Kulczycki LL 1958 Long-term study of one hundred five patients with cystic fibrosis: studies made over a five- to fourteen-year period. J Dis Child 96: 6–15
Moore BJ, Durie PR, Forstner GG 1985 The assessment of nutritional status in children. Nutr Res 57: 97–9
Boyum A 1968 Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Invest 97: 77–89
Cohen SA, Bidlingmeyer BA, Tarvin TL 1986 PITC derivatives in amino acids analysis. Nature 320: 769–770
Fukuda K, Hirai Y, Yoshida H, Nakajima T, Usui T 1982 Free amino acid content of lymphocytes and granulocytes compared. Clin Chem 8: 1758–1761
Parry-Billings M, Evans J, Calder PC, Newsholme EA 1990 Does glutamine contribute to immunosuppression after major burns?. Lancet 336: 523–525
Askanazi J, Carpentier YA, Michelsen CB, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM 1980 Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg 192: 78–85
Parry-Billings M, Baigrie RJ, Lamont PM, Morris PJ, Newsholme EA 1992 Effects of major and minor surgery on plasma glutamine and cytokine levels. Arch Surg 127: 1237–1240
Karinch AM, Pan M, Lin CM, Strange R, Souba WW 2001 Glutamine metabolism in sepsis and infection. J Nutr 131: 2535S–2538S
Petersson B, Vinnars E, Waller SO, Wernerman J 1992 Long-term changes in muscle free aminoacid levels after elective abdominal surgery. Br J Surg 79: 212–216
Engel JM, Mühling J, Weiss S, Löhr T, Simonis Y, Menges T, Hempelmann G 2003 Low plasma glutamine after multiple trauma: relationship with intracellular glutamine in polymorphonuclear neutrophils during prolonged ICU stay. Acta Anaesthesiol Scand 47: 707–713
Hulsewè KW, Van der Hulst RW, Van Acker BA, Von Meyenfeldt MF, Soeters PB 2004 Inflammation rather than nutritional depletion determines glutamine concentrations and intestinal permeability. Clin Nutr 23: 1209–1216
Yoshimura K, Nakamura H, Trapnell BC, Chu CS, Dalemans W, Pavirani A, Lecocq JP, Crystal RG 1991 Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res 19: 5417–5423
Witko-Sarsat V, Allen RC, Paulais M, Nguyen AT, Bessou G, Lenoir G, Descamps-Latscha B 1996 Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J Immunol 157: 2728–2735
Corvol H, Fitting C, Chadelat K, Jacquot J, Tabary O, Boule M, Cavaillon JM, Clement A 2003 Distinct cytokine production by lung and blood neutrophils from children with cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 284: L997–L1003
Furukawa S, Saito H, Inoue T, Matsuda T, Fukatsu K, Han I, Ikeda S, Hidemura A 2000 Supplemental glutamine augments phagocytosis and reactive oxygen intermediate production by neutrophils and monocytes from postoperative patients in vitro. Nutrition 16: 323–329
Castell L 2003 Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. Sports Med 33: 323–345
Fruhwirth M, Ruedl C, Ellemunter H, Bock G, Wolf H 1998 Flow-cytometric evaluation of oxidative burst in phagocytic cells of children with cystic fibrosis. Int Arch Allergy Immunol 117: 270–275
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant (60%) from the Italian Ministry of University and Scientific and Technological Research.
Rights and permissions
About this article
Cite this article
D'Eufemia, P., Finocchiaro, R., Celli, M. et al. Neutrophil Glutamine Deficiency in Relation to Genotype in Children with Cystic Fibrosis. Pediatr Res 59, 13–16 (2006). https://doi.org/10.1203/01.pdr.0000191139.17987.5a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000191139.17987.5a
This article is cited by
-
Immunonutrition in Acute Respiratory Distress Syndrome
Current Pulmonology Reports (2017)