Abstract
The cause of sudden infant death syndrome (SIDS) is an unresolved problem of high relevance. Previous studies indicate a role of infections. In our prospective study, we investigated the frequency of virus-induced myocardial affections in SIDS. Postmortem samples from SIDS victims and control subjects were investigated prospectively. Pediatric cases of unnatural death served as controls. Samples were studied for enteroviruses, adenoviruses, parvovirus B19, and Epstein-Barr virus applying PCR. Immunohistochemical investigations for inflammatory cells, the necrosis marker C5b-9(m) complement complex, and the enteroviral capsid protein VP1 were performed. Overall, 62 SIDS victims were studied. As controls, 11 infants were enrolled. Enteroviruses were detected in 14 (22.5%), adenoviruses in 2 (3.2%), Epstein-Barr viruses in 3 (4.8%), and parvovirus B19 in 7 (11.2%) cases of SIDS. Control group samples were completely virus negative. Compared with controls, immunohistochemical investigations partially revealed a significant increase in the number of T lymphocytes in SIDS myocardial samples (p < 0.05). Furthermore, cases with elevated numbers of leukocytes and + necroses, and enteroviral VP1 macrophages, microfocal C5b-9(m) capsid protein within the myocardium were detected. Applying a comprehensive combination of molecular and immunohistochemical techniques, our results demonstrate a clearly higher prevalence of viral myocardial affections in SIDS. Our results emphasize the importance of PCR-based diagnosis of viral myocardial affections. We suggest preliminary criteria for cellular immunohistochemical diagnosis of viral myocardial affections derived from our findings. For future investigations in SIDS, we suggest a comprehensive approach that includes PCR and immunohistochemistry. Our results offer novel strategies for diagnosis of pediatric myocardial viral affections.
Similar content being viewed by others
Main
The diagnosis of sudden infant death syndrome (SIDS) is established by comprehensive exclusion of all other possible causes of death in the age group. Myocarditis is a widely known explanation for sudden death in cases of suspected SIDS as well as in older children (1). Recent clinical studies and anecdotal communications reported on different viruses in such cases applying molecular techniques to detect the genome sequences of enteroviruses (EV) (2), adenoviruses (AV) (3), Epstein-Barr virus (EBV) (4), and parvovirus B19 (PVB19) (5) in clinical and autopsy samples, respectively. Until recently, a maximum percentage of up to 17% of cases of sudden unexpected death in infancy showing—if at all— histologic hints of viral myocardial affections was assumed (6). The aim of our study was to determine the incidence of virus-induced myocardial affections in cases of SIDS with modern immunohistochemical and molecular pathologic methods.
METHODS
Postmortem samples.
In this prospective study, postmortem tissue samples were obtained in from 1996 to 2001. Initially, 74 cases were studied; in 12 cases, a plausible cause of death [e.g. pneumonia, meningitis, myocarditis according to the Dallas criteria (7)] was revealed by macroscopic or conventional histologic investigations. All slides were investigated by two independent blinded investigators. Subsequently, 62 SIDS cases (study group) without any relevant pathologic findings and 11 cases of unnatural deaths—traffic accident (n = 4), home accident (n = 3), stabbing (n = 2), and drowning (n = 2)—aged 2 to 11 mo (control group) were enrolled (Table 1). Partially, investigations were conducted to verify the cause of death upon instruction by the prosecution authorities; in all other cases, informed consent was obtained from the relatives.
Together with routine autopsy samples from all internal organs, eight samples were taken from standardized locations of each heart (right ventricle anterior and posterior, ventricular septum cranial and caudal, left ventricle anterior wall and posterior wall, cranial and caudal) and from liver and spleen. As fixative neutral phosphate-buffered formaldehyde (pH 7.0) was used, the samples were fixed for up to 48 h. Sections (5 μm) from all samples were stained with hemalaun-eosin.
Sample preparation and PCR procedures.
Sections from myocardial, liver, and spleen samples were investigated for enteroviral RNA including Coxsackie virus B3 RNA (Fig. 1) and several viral DNA [AV, EBV (Fig. 2), PVB19] by (semi-) nested reverse transcriptase–PCR (RT-PCR) and PCR, respectively. Nucleic acids were isolated from paraffin-embedded samples applying techniques published elsewhere (8). Control PCR amplification to verify the presence of amplifiable nucleic acid extracted from each sample was performed using modified cyclophilin primers (9). Primers and thermal conditions used for amplification of virus-specific sequences and the housekeeping gene cyclophilin are shown in Tables 2 and 3. Amplified PCR products were analyzed on 8% polyacrylamide gels; DNA fragments were detected by silver staining as published previously (8). For avoiding false-positive results from contamination, preventive measures were followed (10) and negative controls were performed in all experiments. For each assay, known positive controls, derived from infected viral cells, were added. Purified PCR products were sequenced on an automated ABI 310 sequencer. Sequence comparison was performed by BLAST search of National Center of Bio-technology Information GenBank database.
Immunohistochemistry.
The methods applied for immunohistochemical stainings and antibodies used have been described in detail elsewhere (8, 11). For visualization of all antibodies, a labeled streptavidin-biotin technique was used; 3-amino-9-ethylcarbazol was applied as chromogen. The sections were counterstained with Mayer's hemalaun. In all tests, a negative control was performed and the primary antibody was replaced with diluent only. Human tonsil tissue was used for positive controls.
Leukocytes, T lymphocytes, and macrophages were counted in 20 randomized high-power fields (HPF; magnification ×400), and the mean value was calculated. In contrast to criteria for adults (12, 13), more rigorous criteria were applied for the diagnosis of myocardial affections in infants, discussed in detail below.
A lymphocytic infiltration, i.e. myocarditis, was defined as a mean value of >10 lymphocytes/HPF or a leukocytic infiltration of >15 leukocytes/HPF. Mean values of 5–9 T lymphocytes and >10 macrophages/HPF were regarded as “suspicious.”
Statistical analysis was performed with Wilcoxon's and t tests (SPSS version 10.0). A p < 0.05 was deemed significant.
Toxicologic investigations.
The autopsies were complemented with toxicologic analyses for ethanol and medical and illicit drugs in all cases.
RESULTS
Molecular investigations.
The results of the molecular investigations are listed in Table 4. One case with immunohistochemically detectable marked cellular infiltration and negative results for the viruses investigated so far was diagnosed as human herpes simplex virus type 6 (HHSV6)-induced myocarditis by external investigations. In total, myocardial samples from 27 (43.5%) of 62 SIDS cases were virus positive for one of the viruses investigated. In these cases, viral genome was detectable in some but not all eight myocardial samples taken at autopsy. No cases of double infection were found. All myocardial samples from the control group were found to be virus negative. Moreover, all liver and spleen samples from both groups investigated were found to be virus negative, indicating an isolated myocardial localization of the viruses detected.
Immunohistochemistry.
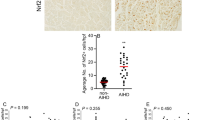
In myocardial slides investigated, leukocytes, T lymphocytes, and macrophages were found in diffuse distribution patterns and occasionally as microfocal accumulations (Fig. 3). Within the study group, four cases exhibited microfocal C5b-9(m)+ myocardial necroses. Microfocal expression of enteroviral VP1 capsid protein was detected in six SIDS cases (Fig. 4). Neither C5b-9(m)+ myocardial necroses nor enteroviral VP1 capsid protein were found in myocardial samples of the control group.
Twelve cases showed immunohistochemical signs of inflammation but negative PCR results for the viruses investigated. Seven PCR-diagnosed virus-positive cases with accompanying immunohistochemical signs of inflammation were found. Five EV cases were diagnosed; four of these EV cases showed T-lymphocytes counted as “suspicious,” and one case exhibited >10 T-lymphocytes and was diagnosed as “sign of active myocarditis” by immunohistochemistry. In addition, one HHSV6 and one PVB19 case were found; both were assessed as “suspicious” because of their elevated T-lymphocyte count. A third PVB19-positive case was diagnosed as “macrophage-rich inflammatory process.” A fourth one presented >15 leukocytes/HPF.
Significant differences were found with regard to the counted mean values of T-lymphocytes in the study group in comparison with the control group (p < 0.05)
Conventional histologic analysis and toxicologic investigations.
In tissue sample slides from internal organs, no relevant pathologic findings were found. Fourteen cases from the study group showed slight bronchitis, all cases with pulmonary edema and hepatic congestion. Cases from the control group also revealed no relevant pathologic findings. Medicolegal toxicologic investigations were completely negative in both groups.
DISCUSSION
The present study for the first time proves evidence of a more than doubled incidence of virus-induced myocardial affections in SIDS than originally assumed (43.5%versus 16.8%). These important findings should have a marked impact on the quality of future pediatric-pediopathologic and medico-legal investigations in such cases. Most important, the results presented and the investigation techniques and criteria suggested here will enable physicians to clarify the cause of death in an increased number of cases of suspected SIDS.
Up to now, only case series and small morphologic studies reported on inflammatory myocardial processes in SIDS victims applying molecular pathologic and immunohistochemical methods to detect viral genome (8, 14, 15). The results of these studies already pointed toward a possible myocardial affection as cause of death, most probably as a result of viral infection. In accordance with these findings, EV were found to be the most common causal agents of myocarditis with seasonal accumulation. Further evidence came from reports of elevated incidence of sudden cardiac death during enterovirus epidemics, particularly CVB3 (16, 17).
Moreover, in adults, virus detection by RT-PCR and in situ hybridization as well as serologic studies detecting viral antibodies revealed an association between enterovirus infection, especially the cardiotropic Coxsackie group B viruses and myocarditis (2). Coxsackie B viruses are one of the most frequently identified infectious agents in acute myocardial infections. Recently, a cDNA clone that encodes the common Coxsackie-adenoviral-receptor was discovered (18). Intriguing is that this receptor has been shown to be down-regulated after birth. The Coxsackie-adenoviral-receptor protein is not detectable in adolescents and adults but was found to be expressed in cases of chronic inflammatory cardiomyopathies in adults. Therefore, this observation offers a feasible molecular basis for the explanation of the observed predominance of the above-mentioned viruses in the heart.
Although there are reports about the myocytopathic effect of enteroviruses in vitro and in animal models (19), the underlying pathomechanism of myocardial damage in humans has remained partially unsolved up to now. It was demonstrated in vitro that enteroviral protease 2A cleaves and therefore functionally impairs dystrophin, a cytoskeletal protein of cardiomyocytes. During infection with Coxsackie B3 viruses, the dystrophin-glycoprotein complex therefore becomes disrupted and the sarcolemmal integrity is lost (20). To us, these results lead to the conclusion that the detection of enteroviral genome within the myocardium is itself a pathologic finding.
However, for the other viruses investigated in our study, the pathomechanisms still remain elusive and are a matter of great scientific interest. In clinical practice, however, it is widely recognized that EBV and HHSV, for example, are without doubt cardiopathogenic agents when detected in endomyocardial biopsy samples. Furthermore, recent studies have demonstrated a correlation between outcome of a coxsackievirus infection and immune status of the host. SIDS victims are often premature infants and mainly die between 8 and 16 wk of age, as the level of maternal antibodies declines. Although the clinical relevance of PVB19 has not been elucidated completely yet, there is an increasing amount of reports on PVB19-induced lethal myocarditis in children (5, 21).
As a matter of fact, all viruses investigated in our prospective study were detected exclusively in myocardial samples, whereas spleen and liver samples were found to be virus negative without exception, indicating an isolated affection of the myocardium in SIDS victims. This is underlined by the fact that all tissue samples from the control group were completely virus negative. Previous studies with histologic investigation of myocardial lesions in cases of SIDS based on conventional stainings revealed only unspecific findings (6), and diagnosis often is not reliable because of considerable interobserver variability (22).
In our prospective study, we performed molecular investigations in combination with immunohistochemistry and conventional light microscopy, offering a new and comprehensive approach to virus detection in myocardial and tissue samples, respectively. As a first step, a defined spectrum of viruses was investigated by PCR, which have been shown to cause myocarditis in infants and adults (3, 5, 15).
With regard to the time-dependent course of viral myocarditis, as studied in a mouse model, early virus-induced myocardial damage already takes place before histologic and immunohistochemical signs of myocarditis defined by the Dallas criteria can be observed (23, 24). These early-phase-dependent viral lesions can be detected only via electron microscopy; they also occur before immunohistochemical signs of myocarditis (19). This is confirmed by our findings, as only four cases of the study group were showed microfocal necroses applying the early necrosis marker C5b-9(m). Six cases of SIDS were stained positive for the enteroviral capsid protein VP1; most intriguing, these all were confirmed as virus positive by RT-PCR for EV RNA. However, according to our observations, an infiltration of leukocytes can be observed by immunohistochemistry before myocardial necroses develop.
In the past, using conventional histologic stainings, a mean value of >5 T lymphocytes/HPF has been regarded as a sign of active myocarditis in adults (13). Other authors suggested an upper normal limit of at least >10 lymphocytes and macrophages per HPF for this diagnosis (12, 25). In our prospective study, we observed great differences in the number of the cells that might reflect normal interindividual variability in infancy. Given the abovementioned discussion on a diagnostic criterion for myocarditis based on the number of inflammatory cells per HPF, we would like to suggest the following preliminary criteria for cellular immunohistochemical diagnosis of viral myocardial affections. In contrast to criteria for analysis of adult samples, we have chosen more rigorous criteria to ensure the quality of diagnosis in infants. These suggestions are derived from our findings, especially with respect to the analysis of the control group. More than 10 T-lymphocytes/HPF (as observed in 6 of 62 SIDS cases) should be interpreted as a reliable sign of active myocarditis. Furthermore, cases that show >15 T lymphocytes and macrophages in summation should also be diagnosed as “active myocarditis ” (6 of 62 SIDS cases). Cases with 5 to 10 T-lymphocytes/HPF (11 of 62 SIDS cases) should be regarded as “suspicious.” In addition, we found five cases with >10 CD68+ macrophages/HPF accompanied by <5 T-lymphocytes, resulting in a total of <15 cells as postulated above. This phenomenon remains unclear at the moment; however, it may indicate a late inflammatory process (e.g. resolving myocarditis). Therefore, we would like to suggest the preliminary term “macrophage-rich inflammatory process.” Cases that present with <5 T-lymphocytes or 10 macrophages should be assessed as “without pathologic findings ” (as observed in all samples of the control group).
As a matter of fact, 17 of 27 virus-positive cases detected by PCR were observed without any immunohistochemical signs of inflammation according to our new criteria (Table 5). Given the time course of myocarditis development discussed above, these cases might represent a very early and clinical inapparent phase with death preceding any inflammatory cellular reaction. Twelve cases exhibited immunohistochemical signs of inflammation but negative PCR results for the viruses investigated (Table 5). It has to be mentioned here that the total spectrum of cardiotropic viruses includes a larger number than assessed in our study.
The question of whether early viral infection can indeed cause lethal myocardial damage has been an issue of scientific debate in the past. Depending on the localization, a virus-induced inflammatory process that affects the myocardial conduction system already at an early stage must be considered as a possible cause of sudden death not only in infants. With regard to that issue, clinical studies as well as case reports describe enteroviral, adenoviral, and EBV-induced myocarditis characterized by a fulminant clinical course with malignant arrhythmias (17).
CONCLUSION
In synopsis, the results from our prospective study on the role of virus-induced myocardial affections in unexpected SIDS indicate that the incidence of potentially lethal viral myocardial affections is more than twice than assumed up to now. However, our results should be regarded as a part of numerous findings pointing toward an underlying inflammatory process in cases of SIDS. After exclusion of other possible causes of death, our results indicate that viral myocardial affection is the cause of death in EV, AV, EBV, and HHSV6-positive SIDS cases; moreover, PVB19 seems to play a more critical role than assumed so far.
Our results prove the importance of combined investigations using molecular pathologic techniques and immunohistochemical methods. We suggest new accurate sampling standards as well as novel diagnostic criteria for future investigations. These standards and criteria should enable pathologists to establish more reliable diagnoses of myocarditis in pediatric fatalities and therefore derive possible future clinical therapeutic strategies (26).
Abbreviations
- AV:
-
adenovirus
- C5b-9(m):
-
complement complex C5b-9(m)
- CVB3:
-
coxsackievirus B3
- EBV:
-
Epstein-Barr virus
- EV:
-
enterovirus
- HHSV6:
-
human herpes simplex virus type 6
- HPF:
-
high-power field
- PVB19:
-
parvovirus B 19
- RT-PCR:
-
reverse transcriptase–PCR
- SIDS:
-
sudden infant death syndrome
- VP-1:
-
viral protein 1
References
Rasten-Almqvist P, Eksborg S, Rajs J 2002 Myocarditis and sudden infant death syndrome. APMIS 110: 469–480.
Jin O, Sole MJ, Butany JW, Chia WK, McLaughlin PR, Liu P, Liew CC 1990 Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation 82: 8–16.
Lozinski GM, Davis GG, Krous HF, Billman GF, Shimizu H, Burns JC 1994 Adenovirus myocarditis: retrospective diagnosis by gene amplification from formalin-fixed, paraffin-embedded tissues. Hum Pathol 25: 831–834.
Hebert MM, Yu C, Towbin JA, Rogers BB 1995 Fatal Epstein-Barr virus myocarditis in a child with repetitive myocarditis. Pediatr Pathol Lab Med 15: 805–812.
Murry CE, Jerome KR, Reichenbach DD 2001 Fatal parvovirus myocarditis in a 5-year-old girl. Hum Pathol 32: 342–345.
Rambaud C, Cieuta C, Canioni D, Rouzioux C, Lavaud J, Hubert P, Brousse N, Rudler M, Cheron G 1992 Cot death and myocarditis. Cardiol Young 2: 266–271.
Aretz HT 1987 Myocarditis: the Dallas criteria. Hum Pathol 18: 619–624.
Dettmeyer R, Baasner A, Schlamann M, Haag C, Madea B 2002 Coxsackie B3 myocarditis in 4 cases of suspected sudden infant death syndrome: diagnosis by immunohistochemical and molecular-pathologic investigations. Pathol Res Pract 198: 689–696.
Ben-Ezra J, Johnson DA, Rossi J, Cook N, Wu A 1991 Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem 39: 351–354.
Kwok S, Higuchi R 1989 Avoiding false positives with PCR. Nature 339: 237–238.
Li Y, Bourlet T, Andreoletti L, Mosnier JF, Peng T, Yang Y, Archard LC, Pozzetto B, Zhang H 2000 Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation 101: 231–234.
Linder J, Cassling RS, Rogler WC, Wilson JE, Markin RS, Sears TD, McManus BM 1985 Immunohistochemical characterization of lymphocytes in uninflamed ventricular myocardium. Arch Pathol Lab Med 109: 917–920.
Edwards WD, Holmes DR, Reeder GS 1982 Diagnosis of active lymphocytic myocarditis by endomyocardial biopsy: quantitative criteria for light microscopy. Mayo Clin Proc 57: 419–425.
Dettmeyer R, Schlamann M, Madea B 1999 Immunohistochemical techniques improve the diagnosis of myocarditis in cases of suspected sudden infant death syndrome (SIDS). Forensic Sci Int 105: 83–94.
Shimizu H, Rambaud C, Cheron G, Rouzioux C, Lozinski GM, Rao A, Stanway G, Krous HF, Burns JC 1995 Molecular identification of viruses in sudden infant death associated with myocarditis and pericarditis. Pediatr Infect Dis J 14: 584–588.
Phillips CA, Aronson MD, Tomkow J, Phillips ME 1980 Enteroviruses in Vermont, 1969–1978: an important cause of illness throughout the year. J Infect Dis 141: 162–164.
Mounts AW, Amr S, Jamshidi R, Groves C, Dwyer D, Guarner J, Dawson JE, Oberste MS, Parashar U, Spevak P, Alexander J 2001 A cluster of fulminant myocarditis cases in children, Baltimore, Maryland, 1997. Pediatr Cardiol 22: 34–39.
Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW 1997 Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275: 1320–1323.
Kandolf R, Klingel K, Zell R, Canu A, Fortmuller U, Hohenadl C, Albrecht M, Reimann BY, Franz WM, Heim A et al. 1993 Molecular mechanisms in the pathogenesis of enteroviral heart disease: acute and persistent infections. Clin Immunol Immunopathol 68: 153–158.
Badorff C, Berkely N, Mehrotra S, Talhouk JW, Rhoads RE, Knowlton KU 2000 Enteroviral protease 2A directly cleaves dystrophin and is inhibited by a dystrophin-based substrate analogue. J Biol Chem 275: 11191–11197.
Dettmeyer R, Kandolf R, Baasner A, Banaschak S, Eis-Hubinger AM, Madea B 2003 Fatal parvovirus B19 myocarditis in an 8-year-old boy. J Forensic Sci 48: 183–186.
Shanes JG, Ghali J, Billingham ME, Ferrans VJ, Fenoglio JJ, Edwards WD, Tsai CC, Saffitz JE, Isner J, Furner S et al. 1987 Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation 75: 401–405.
Feldman AM, McNamara D 2000 Myocarditis. N Engl J Med 343: 1388–1398.
Cioc AM, Nuovo GJ 2002 Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod Pathol 15: 914–922.
Cassling RS, Linder J, Sears TD, Waller BF, Rogler WC, Wilson JE, Kugler JD, Kay DH, Dillon JC, Slack JD, McManus BM 1985 Quantitative evaluation of inflammation in biopsy specimens from idiopathically failing or irritable hearts: experience in 80 pediatric and adult patients. Am Heart J 110: 713–720.
Rotbart HA, Webster AD, Pleconaril Treatment Registry Group 2001 Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis 32: 228–235.
Martin AB, Webber S, Fricker FJ, Jaffe R, Demmler G, Kearney D, Zhang YH, Bodurtha J, Gelb B, Ni J, Bricker T, Towbin JA 1994 Acute myocarditis. Rapid diagnosis by PCR in children. Circulation 90: 330–339.
Klump WM, Bergmann I, Muller BC, Ameis D, Kandolf R 1990 Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5′ uridine residues are regained during plus-strand RNA synthesis. J Virol 64: 1573–1583.
Chang KL, Chen YY, Chen WG, Hayashi K, Bacchi C, Bacchi M, Weiss LM 1999 EBNA-1 gene sequences in Brazilian and American patients with Hodgkin's disease. Blood 94: 244–250.
Cassinotti P, Weitz M, Siegl G 1993 Human parvovirus B19 infections: routine diagnosis by a new nested polymerase chain reaction assay. J Med Virol 40: 228–234.
Acknowledgements
The authors are indebted to U. Cremer and K.H. Schiwy-Bochat for support. We thank H. Langer, C. König, H. Körner, and R. Klemmer for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Deutsche Forschungsgemeinschaft (DE 814).
Rights and permissions
About this article
Cite this article
Dettmeyer, R., Baasner, A., Schlamann, M. et al. Role of Virus-Induced Myocardial Affections in Sudden Infant Death Syndrome: A Prospective Postmortem Study. Pediatr Res 55, 947–952 (2004). https://doi.org/10.1203/01.pdr.0000127022.45831.54
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000127022.45831.54
This article is cited by
-
Diagnostic challenges and forensic implications in a case of infantile fatal myocarditis
Forensic Science, Medicine and Pathology (2023)
-
Letale lymphozytäre Myokarditis – eine unterschätzte Diagnose im Säuglings- und Kindesalter?
Die Pathologie (2023)
-
Sudden cardiac death—update
International Journal of Legal Medicine (2021)
-
Postmortale Diagnostik einer (post-)viralen Myokarditis bei mutmaßlichem plötzlichem Säuglingstod
Rechtsmedizin (2016)
-
Immunohistochemical diagnosis of myocarditis on (infantile) autopsy material: Does it improve the diagnosis?
Forensic Science, Medicine, and Pathology (2015)