Abstract
The metabolic derangements in severe protein-energy malnutrition (PEM) are only partially known, due to the limitations of blood collection in these patients. Urinary excretion of organic acids was studied by gas chromatography-mass spectrometry in 39 infants with four types of PEM:1) upon hospital admission, as soon as eventual infections had been cleared, and salt and water deficits corrected, but before oral feeding was started; 2) after start of protein alimentation;3) on the day of discharge. All of the patients showed an increased excretion of various organic acids at some point of their hospital stay, regardless of the clinical type of PEM. In nearly half of the malnourished children, results were suggestive of blocks in the pathways of propionate (15.4% with increased methylmalonate and 25.6% with 2-methylcitrate), of fatty acid β-oxidation (30.8% with raised dicarboxylic acids with low or low normal 3-hydroxybutyrate), or of both pathways (12.8%). These abnormalities may have been caused by cofactor deficiencies (biotin, vitamin B12, riboflavin, carnitine, niacin). Dicarboxylic acids were excreted in high amounts since the initial sample, probably due to increased mobilization of fatty acids. Increased 2-methylcitrate and methylmalonate excretion was observed more frequently once patients started to be orally fed. The accumulation of potentially toxic acyl-CoA precursors of these compounds could contribute to the known clinical worsening of some malnourished infants after suddenly increased protein intake. Other less specific metabolites, such as 3-hydroxybutyrate, lactate, 4-hydroxyphenyllactate, fumarate, succinate, and 4-hydroxyphenylacetate, were also abnormally excreted in some patients. The analysis of urinary organic acids provides a new approach for the metabolic study of PEM and may have diagnostic and therapeutic implications.
Similar content being viewed by others
Main
Analysis of urinary organic acids by GCMS has revealed a whole new spectrum of metabolic abnormalities in the monogenic genetic diseases, the so-called organic acidurias(1). Organic acids are defined as non-amino carboxylic acids, and include many compounds of carbohydrate (e.g. lactic acid), lipid (fatty acids), and amino acid (many of their catabolic intermediates) metabolism(2). More than 100 compounds can be studied in a single sample. Therefore, their analysis is a powerful adjunct to the study of metabolic derangements, with the advantage of it being performed on urine, an easy to obtain material. Severe infant PEM is a frequent disorder in developing countries and, although it has been studied for decades, many metabolic abnormalities are still not well known, because of the difficulties of obtaining blood samples from very small and sick children. We hypothesized that the study of organic acids by GCMS in urine from these infants might reveal previously unknown abnormalities. In this study, we report that there is indeed a substantial prevalence of abnormalities of these compounds in PEM.
METHODS
Patients. Thirty-nine patients with severe PEM admitted at Mexico's National Institute of Pediatrics were studied: 9 with classical kwashiorkor, 10 with marasmus, and 10 with marasmic kwashiorkor, in terms of the Wellcome classification(3). The other 10 patients were classified as "sugar babies." The latter term was coined by Platt(4) to describe malnourished infants with a body weight of 60-80% normal for age, edema, and some s.c. adipose tissue, but with only minimal changes in the skin and hair that he thought were produced by low protein, high carbohydrate diets. Clinical history, physical examination, and routine laboratory tests were performed, including nutritional assessment. Body weight, height, and head circumference were measured as recommended by Jeliffe(5). Normal reference data for Mexican children were those reported by Ramos-Galván(6). A summary of the patient data is shown in Table 1. Children were excluded or eliminated if they were concurrently affected with another disease besides malnutrition, such as intestinal ischemia, liver or kidney failure, or if they had received any vitamins, carnitine, valproic acid, tetracyclines, blood transfusions, or parenteral alimentation. They were also eliminated if infection had not subsided by the 5th d after admission, or if they could not be orally fed. Informed consent was granted in all cases by parents or legal guardians. The control group consisted of 70 eutrophic children whose height and weight were between the 5th and the 95th percentile. This research protocol was previously approved by the Research and Ethics Committees of the National Institute of Pediatrics.
Experimental design. The patients were investigated on three occasions: 1) on the first 24-72 h after hospital admission, once overt infection, dehydration, and metabolic acidosis had been controlled, but before protein feeding was started (initial sample); 2) between the 5th and 20th d of the study (median, d 8), after oral alimentation had started (intermediate sample); and 3) at the time of discharge from the hospital (21-82 d; median, 34), once the weight to height deficit was less than 14% (final sample). Initially, the patients' formula was made of calcium caseinate, glucose, corn oil, and potassium chloride, and it did not contain added milk nor vitamins. Increasing amounts of powdered milk (starting with 8 g/L) were added to the formula; all the patients tolerated the formula well. The patients had a normal diet at the time of the final study. At each occasion, a random urine sample was collected and immediately frozen at -20°C; care was taken to avoid specimen contaminated by feces or by secretions from the rash that some patients presented. All the samples were obtained early in the morning, before the first feeding. The recovery period lasted from 3 to 9 wk; infants with marasmus and marasmic kwashiorkor had a more protracted recovery and stayed in the hospital for a substantially longer time. For administrative reason nine patients (two kwashiorkor, two marasmic kwashiorkor, and five"sugar babies") were transferred to another hospital before they had achieved a weight to height deficit lower than 14%, and no final sample could be obtained from them.
Assays. Urinary organic acids were determined by GCMS after solid phase extraction by a method developed by us. An internal standard, 1,12-dodecanedioic acid (Sigma Chemical Co., St. Louis, MO), was added to a urine volume containing 0.5 µmol of creatinine. The sample was then acidified with 200 µL of concentrated HCl. The extraction column(Silica Gel Speed, SPE cartridges, Allentown, PA) containing 1.0 g was conditioned with 4 mL of HCl 0.1 M in methanol, and dried under vacuum; 4 mL of H2SO4 50 mM in methanol were then added and the column was again vacuum-dried. One sample per cartridge was applied on the top of the column using gentle suction. The column was dried under vacuum for 15 min, and the organic acids were eluted with 2 mL of 20%t-butanol/chloroform. The eluate was centrifuged and evaporated to dryness. Trimethylsilyl derivatives were formed by adding 100 µL of N,O-bis-(trimethylsilyl)trifluoroacetamide (Supelco, Bellefonte, PA) and heating the samples for 30 min at 60°C. GCMS analysis was performed on a fused silica capillary column 25 m × 0.25-mm inside diameter, containing a 0.25-µm thick film of methyl 50% phenyl silicone(Quadrex, New Haven, CT). The GCMS system consisted of a Hewlett-Packard HP 5890 series II gas chromatograph and a MSD 5971 mass spectrometer with a workstation (HP Chem Station). The chromatographic parameters were injected on the splitless mode at 250°C, the injected samples 1 µL, the initial temperature at 50°C for 5 min, increased to 280°C at 10°C/min, and kept at 280°C for 7 min. Helium was used as carrier gas with a flow rate of 1 mL/min. The mass spectrometer was operated at the scan mode; the temperature of transfer line was kept at 280°C; scan range from 50 to 650 atomic mass units, solvent delay 10 min. After acquisition of the data, the organic acids were identified using a mass spectra library kindly given to us by Dr. Lawrence Sweetman. Compounds were quantified from the areas of extracted ion chromatograms of ions unique (or nearly unique) for each compound, effectively increasing the chromatographic resolution of overlapping peaks in the total ion chromatogram(7,8).
The determination of urinary organic acids by GCMS is a method of great specificity, but has some limitations as a quantitation procedure for such a heterogenous group of compounds as the urinary organic acids, resulting in the well known wide normal ranges(2). Ideally, the results would be expressed in terms of 24-h urine collections. In practice, these samples are difficult to obtain in severely malnourished infants, due to the presence of frequent rashes in the perineal region, especially on admission. In this work, we used random urine specimens and expressed the results in terms of the creatinine content of the samples, to correct for the variable concentration of the urine samples, as is usually done(2,9,10). Although there was some tendency to lower urinary creatinine concentrations in the initial and intermediate samples, compared with the final ones, several different acids(fumaric, 3-hydroxybutyric, ethylmalonic, 4-hydroxyphenylacetic, and benzoic) did not show statistically significant differences per creatinine for the three different collection periods. Moreover, when results were abnormal, the elevations were found only for isolated acids in particular infants.
RESULTS
All of the 39 patients studied had an initial or intermediate abnormal urinary excretion, defined as a value above the normal control group or the normal values in American infants reported by Sweetman(2), whichever were the higher (Table 2,Figs. 1–3). Some of the final values were higher than those of our own normal samples (Table 2) or those of previously published ones.
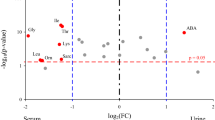
Urinary 2-methylcitrate concentrations of PEM infants on three occasions: initial, on the first 24-72 h after hospital admission, once overt infection, dehydration and metabolic acidosis had been controlled, but before protein feeding was started;intermediate, between the 5th and 20th d of the study (median, d 8), after oral alimentation had started; and final, at the time of discharge from the hospital (21-82 d; median, 34), once the weight-to-height deficit was less than 14%. The control group consisted of 70 eutrophic children whose height and weight were between the 5th and 95th percentile.
There was great heterogeneity of the abnormalities present in the different individual patients. Furthermore, there was no recognizable pattern of abnormalities associated with a particular clinical type of malnutrition.Table 3 shows the frequency of patients with elevations of selected organic acids; this selection was based either on their suitability as markers of metabolic blocks, on their potential origin from bacterial metabolism, or on their high levels in some of the patients.
The compounds more frequently raised were lactic and 4-hydroxyphenyllactic acids. Others were 2-methylcitrate, methylmalonate, 3-hydroxybutyrate, dicarboxylic acids (adipic, suberic, and sebacic), fumarate, succinate, benzoate, and 4-hydroxyphenylacetate. Most abnormalities were present on admission. However, in the case of increased 2-methylcitrate and, especially, methylmalonate, they tended not to increase above normal in the initial, but in the intermediate sample (Table 3, Figs. 1 and 2).
DISCUSSION
Our study shows, for the first time as far as we are aware, that most, if not all, infants with severe PEM exhibit organic acidurias. In this study, the condition usually disappeared in the final sample; however, some of these values continued being higher than those of our own normal samples(Table 2) or previously published ones(2), likely reflecting incomplete metabolic recovery at the time of discharge(11). The great diversity of abnormalities in different individual patients and the lack of associations of excretion patterns with the clinical types of PEM, points to the multifactorial and heterogeneous nature of this nutritional disorder.
Several of the abnormally excreted organic acids are intermediates (or their derivatives) of metabolic pathways, and their increased urinary excretion may reflect their accumulation due to blocks in those pathways. In particular, excretion of 2-methylcitrate is specifically related to propionyl-CoA carboxylase deficiency and methylmalonate is excreted in large amounts in methylmalonyl-CoA mutase deficiency, both on the propionate pathway(12). The dicarboxylic aciduria (adipic, suberic, and sebacic), when accompanied by normal or low levels of 3-hydroxybutyrate, may be the result of blocks at the mitochondrial fatty acid β-oxidation pathway(13). Their frequency in our sample was 15.4% for methylmalonate, 25.6% for 2-methylcitrate, and 30.8% for dicarboxylic acids (Table 3, Figs. 1–3). Thus, nearly half of the malnourished infants may have blocks in the propionate or the β-oxidation pathways. Five patients(12.8%) showed a high excretion of dicarboxylic acids together with methylmalonate and/or 2-methylcitrate, possibly indicating simultaneous blocks of the propionate and the β-oxidation pathways. A diagnostic implication of this interpretation is that blocks in these pathways might be detected in severely undernourished patients through the study of urine, an easy-to-obtain sample.
Several steps in these two pathways require cofactors, that themselves or their precursors are vitamins, adenosyl cobalamine for methylmalonyl-CoA mutase, biotin for propionyl-CoA carboxylase, riboflavin, niacin, and carnitine for several of the enzymes of β-oxidation(14). Because vitamin deficiencies frequently occur in PEM, they might have caused the proposed metabolic blocks resulting in the observed organic acidurias. Indeed, there are published reports of abnormal organic acid urinary excretion in isolated deficiencies of some of these vitamins(11,15,16,18–24). We are currently looking for associations between these acidurias and their corresponding vitamin levels in malnourished infants, and whether both respond to the specific vitamin administration. These organic acidurias also occur in some inborn errors of metabolism, and therefore, our findings in PEM may represent nutritional phenocopies of some of these genetic disorders(25).
In the case of a putative biotin deficiency, we did not detect 3-hydroxyisovaleric acid nor 3-methylcrotonylglycine (products of leucine degradation) in our samples. These compounds may have been present but not demonstrated because of our limits of detection. Alternatively, leucine(protein) deprivation might reduce these organic acidurias in spite of a deficiency of the biotin-dependent carboxylase in the leucine pathway(3-methylcrotonyl-CoA carboxylase)(11). A heightened excretion of ethylmalonate, which we observed in five patients(Table 3), has been reported in riboflavin deficiency(15,16) and in the short chain acyl-CoA dehydrogenase deficiency, a β-oxidation defect(17).
There are striking differences between the β-oxidation and the propionate pathways, in relation to the timing of maximal organic acid excretion. It was since admission to the hospital, when the patients were starved, that dicarboxylic acids were excreted in high amounts(Fig. 3, Table 3). Increased mobilization of FFA occurs in this condition(27). If they cannot be thoroughly β-oxidized, a significant proportion of them will be [horizontal bar over omega]-oxidized, giving rise to dicarboxylic aciduria(28).
On the other hand, in most cases 2-methylcitrate and, especially, methylmalonate, were not abnormally excreted initially, but several days later, once the patients started to be orally fed (Figs. 1 and 2, Table 3). Because many of the substrates for the propionate pathway are to some extent of dietary origin(12), their availability may have been limited on admission, when the patients were first tested. The late appearance of abnormal propionate pathway metabolites, with likely cellular accumulation of toxic acyl-CoA intermediates(29), may contribute to the well known but poorly understood worsening of malnourished infants when they are abundantly fed(30).
Metabolic blocks can also be the result of an exogenous toxin, such as hypoglycin, a plant toxin present in unripe ackee fruit, responsible for Jamaica vomiting sickness, a lethal disease resulting in severe hypoglycemia, acidosis, and a complex organic aciduria(31). In the case of our patients, there were no antecedents suggestive of this type of toxic metabolic block.
The rise of other urinary organic acids (Table 3) is less specific; lactate, 3-hydroxybutyrate, fumarate, succinate, 4-hydroxyphenylacetate, or benzoate may not reflect specific metabolic blocks, but rather be the result of the acute illness process that is an inherent part of severe PEM(30). As for dicarboxylic aciduria, it is conceivable that in some of our patients it might have also been unspecific, secondary to a hypothetical mitochondrial damage due to PEM, as has indeed been the case with some primary mitochondrial defects(26).
Although this study refers to primary malnutrition, it can be successfully applied to patients with undernutrition secondary to disorders such as intestinal malabsorption, e.g. celiac disease(32). Thus, urinary organic acid analysis by GCMS represents a novel and powerful noninvasive approach for the study of nutritional disorders.
Abbreviations
- GCMS:
-
gas chromatography-mass spectrometry
- PEM:
-
protein-energy malnutrition
References
Lehotay DC, Clarke JTR 1995 Organic acidurias and related abnormalities, Crit Rev Clin Lab S. ci 32: 377–429.
Sweetman L 1991 Organic acids analysis. In: Hommes FA(ed) Techniques in Diagnostic Human Biochemical Genetics. Wiley-Liss, New York, pp 143–176.
Wellcome Trust Working Party 1970 Classification of infantile malnutrition. Lancet 2: 302–303.
Platt BS 1947 Colonial nutrition and its problems. Trans R Soc Trop Med Hyg 40: 379–398.
Jeliffe B 1968 Protein-energy malnutrition(Desnutrición energético-proteínica) (in Spanish). WHO Monogr Ser 53: 10–101.
Ramos-Galván R 1975 Somatometria pediátrica: estudio longitudinal de niños en la Ciudad de México. Arch Invest Med 6 ( suppl 1): 83–396.
Gates SC, Dendramis N, Sweeley CC 1978 Automated metabolic profiling of organic acids in human urine. I. Description of methods. Clin Chem 24: 1674–1679.
Hoffmann G, Aramaki S, Blum-Hoffmann E, Nyhan WL, Sweetman L 1989 Quantitative analysis for organic acids in biological samples: batch isolation followed by gas chromatographic-mass spectrometry analysis. Clin Chem 35: 587–595.
Chalmers RA, Lawson AM 1982 Organic Acids in Man. Chapman & Hall, New York, pp 182
Goodman SI, Markey SP 1981 Diagnosis of Organic Acidemias by Gas Chromatography-Mass Spectrometry. Alan R Liss, New York, pp 59
Mock DM, Baswell DL, Baker H, Holman RT, Sweetman L 1985 Biotin deficiency complicating parenteral alimentation: diagnosis, metabolic repercussions, and treatment. Ann NY Acad Sci 447: 314–333.
Fenton WA, Rosenberg LE 1995 Disorders of propionate and methylmalonate metabolism. In: Scriver CR, Beaudet AL, Sly W, Valle D(eds) The Metabolic Basis of Inherited Disease. McGraw-Hill, New York, pp 1423–1449.
Stanley CA 1995 Disorders of fatty acid oxidation In: Fernandes J. Saudubray JM, Van den Berghe (eds) Inborn Metabolic Diseases: Diagnosis and Treatment. Springer, Berlin, pp 133–143.
Coombs GF 1992 The Vitamins: Fundamental Aspects in Nutrition and Health. Academic Press, San Diego, CA
Veitch K, Draye JP, Vameq J, Causey AG, Bartlett K, Sherratt HSA, Van Hoof F 1989 Altered acyl-CoA metabolism in riboflavin deficiency. Biochim Biophys Acta 1006: 335–343.
Goodman SI 1981 Organic aciduria in the riboflavin-deficient rat. Am J Clin Nutr 34: 2434–2437.
Roe CR, Coates PM 1995 Mitocondrial fatty acid oxidation disorders. In: Scriver CR, Beaudet AL. Sly WS, Valle D (eds) The Metabolic Basis of Inherited Disease. McGraw-Hill, New York, pp 1523
Cardinale GJ, Dreyfus PM, Auld P, Abeles RH 1969 Experimental vitamin B12 deficiency: its effects on tissue vitamin B12-coenzyme levels and on the metabolism of methylmalonyl-CoA. Arch Biochem Biophys 131: 92–99.
Draye JP, Veitch K, Vamecj, Van Hoof F 1988 Comparison of the metabolism of dodecanedioic acid in vivo in control, riboflavin-deficient and clofibrate-treated rats. Eur J Biochem 178: 183–189.
Geypens B, Ghoos Y, Hiele M, Rutgeerts P, Vantrappen G, Joosten E, Pelemans W 1991 Determination of urinary methylmalonic acid in urine by gas chromatography with an ion-trap detector, chemical ionization and isotope dilution. Anal Chim Acta 247: 243–248.
Norman EJ 1987 New urinary methylmalonic acid test is a sensitive indicator of cobalamin (vitamin B12) deficiency: a solution for a major unrecognized medical problem. J Lab Clin Med 110: 369–370.
Rasmussen K, Moelby L, Jensen MK 1989 Studies on methylmalonic acid in human. II. Relationship between concentrations in serum and urinary excretion, and the correlation between serum cobalamin and accumulation of methylmalonic acid. Clin Chem 35: 2277–2280.
Specker BL, Miller D, Norman EJ, Greene H, Hayes KC 1988 Increased urinary methylmalonic acid excretion in breast-fed infants of vegetarian mothers and identification of an acceptable dietary source of vitamin B-12. Am J Clin Nutr 47: 89–92.
Stabler SP, Marcell PD, Podell ER, Allen RH, Lindenbaum J 1986 Assay of methylmalonic acids in the serum of patients with cobalamin deficiency using capillary gas chromatography-mass spectrometry. J Clin Invest 77: 1606–1612.
Velazquez A 1997 Biotin deficiency in protein-energy malnutrition: implications for nutritional homeostasis and individuality. Nutrition 13: 991–992.
Rabier D, Bardet J, Parvy Ph, Poggi F, Brivet M, Saudubray JM, Kamoun P 1995 Do criteria exist from urinary organic acids to distinguish β-oxidation defects?. J Inherit Metab Dis 18: 257–260.
Newsholme EA, Leech AR 1983 Biochemistry for Medical Sciences. John Wiley & Sons, Chichester, UK, pp 537
Mannaerts GP, Van Veldhoven P 1991 Fatty acid oxidation: general overview. Nestle Nutr Workshop Ser 24: 1–18.
Wendel U, Eissler A, Sperl W, Schadewaldt P 1995 On the differences between urinary metabolite excretion and odd-numbered fatty acid production in propionic and methylmalonic acidaemias. J Inherit Metab Dis 18: 584–591.
Waterlow JC 1994 Childhood malnutrition in developing nations: looking back and looking forward. Ann Rev Nutr 14: 1–19.
Tanaka K, Rosenberg LE 1983 Disorders of branched chain amino acid and organic acid metabolism. In Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, and Brown MS (eds) The Metabolic Basis of Inherited Disease. McGraw-Hill, New York, pp 448–449.
Largilliére C, Fontaine M, Marrakchi S, Voisin-Taboreau O 1993 Pseudo-glutaric aciduria type II in a patient with celiac disease [letter; comment]. J Pediatr 122: 504
Acknowledgements
The authors thank Drs. Silvestre Frenk and Santiago Capella for their generous and effective help.
Author information
Authors and Affiliations
Additional information
Supported by research grant 0599 PM from the Consejo Nacional de Ciencia y Tecnología (CONACYT) of Mexico.
Rights and permissions
About this article
Cite this article
Terán-García, M., Ibarra, I. & Velázquez, A. Urinary Organic Acids in Infant Malnutrition. Pediatr Res 44, 386–391 (1998). https://doi.org/10.1203/00006450-199809000-00020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199809000-00020