Abstract
We determined the placental transfer of TSH in an in vitro model of dually perfused isolated lobule in 28 human term placentas by adding varying concentrations (5-60 μIU mL-1) of TSH as a single bolus dose to the closed maternal circulation. Transmembrane transfer of TSH was also studied by adding 45 μIU mL-1 to the maternal or fetal compartment of a dual chamber of fetal membranes in culture. Passage of freely diffusible markers creatinine and antipyrine were also studied in this model. TSH concentration was measured by third generation chemiluminescence assay with a sensitivity of 10 mIU mL-1. In the perfusion experiments, at physiologic concentrations the slow decline of TSH in the maternal circulation was associated with a small linear increase in fetal levels to 0.11 ± 0.04% of initial dose at 2 h. The placental transfer rate was 0.08 μIU min-1. Increasing maternal concentrations of TSH were associated with proportional increases in transfer rate (y = 0.002x; R2= 0.99) and placental uptake (y = 0.01x; R2 = 0.97). The placental permeability of TSH was 2.4·10-4 mL min-1 g-1 and was proportional to its coefficients of diffusion in water and molecular size. The transmembrane transfer and permeability of TSH was comparable to those of the placenta. We conclude that TSH crosses the human term placenta and fetal membranes sparingly.
Similar content being viewed by others
Main
Respiratory distress syndrome from surfactant deficiency is a major cause of perinatal morbidity and mortality in preterm infants. Antenatal therapy with glucocorticoids to accelerate lung maturation has become standard clinical practice, but reduces the incidence of respiratory distress syndrome by only 50%(1). Animal and human studies suggest that thyroid hormones act additively with glucocorticoids to promote fetal lung maturity. Because thyroxine does not cross the placenta freely(2), clinical interest has focused on antenatal administration of TRH(3) to reduce the incidence of severe respiratory distress syndrome(4).
Recent in vitro data from our group, however, suggest that human term placenta forms an enzymatic barrier to the free passage of TRH(5). Placental clearance of TRH has been shown to be independent of mode of administration, but varies with maternal dose(6). Low fetal levels of TRH found in response to high maternal concentrations are unlikely to be of clinical significance. These findings have been substantiated by an in vivo study in 13 mothers who underwent clinically indicated fetal blood sampling between 24 and 34 wk of gestation(7). More recently, we explored transfer of maternal TRH across the extraplacental chorioamniotic membrane. Similar to the results of placental studies, we found that TRH does not cross the fetal membrane from 24 wk of gestation because the fetal membranes also act as an enzymatic barrier to the bidirectional transfer of TRH(8).
Therefore, the mechanism by which maternal administration of TRH stimulates the fetal thyroid gland remains unclear. One possibility is that maternal TSH crosses the placenta to stimulate the fetal thyroid gland. Although no study has systematically evaluated transplacental transfer of TSH, cord blood data obtained from two infants with congenital absence of the anterior pituitary gland showing very low TSH levels suggest that maternal TSH does not cross the placenta, at least at physiologic levels(9). However, given that most drugs cross the placenta by simple diffusion along a concentration gradient, pharmacologic levels of maternal TSH, such as after TRH administration, may instead cross the placenta.
Alternatively, maternal TSH might enter the fetal compartment via the transmembranous route. Although TSH is present in amniotic fluid from the second trimester(10, 11), little is known of its source. Amniotic fluid TSH has been assumed to be fetal in origin because its levels correlate with cord TSH levels(10). In contrast, the presence of high levels of TSH in the amniotic cavity of anencephalic fetuses(12) suggests instead a maternal origin. Observational in vivo studies showing high mannitol and inulin concentrations in amniotic fluid after maternal administration suggest that hydrophilic substances may enter the amniotic cavity independently of the fetal-placental unit(13, 14). This raises the possibility of transmembraneous transfer of TSH from the maternal to fetal compartments.
The aim of this study was to investigate maternal to fetal transfer of TSH across the perfused human term placenta over a concentration range similar to that attained after maternal administration of 400 μg of TRH i.v. We also investigated the passage of TSH across the fetal membranes in culture at a concentration equivalent to the peak maternal TSH in response to TRH.
METHODS
Analytical grade TSH, creatinine, and TC-199 perfusion media were purchased from Sigma Chemical Co. Chemicals (Poole, Dorset, UK). All other reagents and chemicals were of analytical grade and obtained from BDH (Leicestershire, UK).
Placental perfusion technique. Placentas were obtained immediately after vaginal or cesarean delivery from normal pregnancies between 37 and 42 wk of gestation. Perfusion of the isolated lobule was commenced within 5 min at 37°C under optimal physiologic conditions of oxygenation, pressure, flow, osmotic pressure, and acid/base status(15). In contrast to our previous work on related topics where we used washed maternal and fetal blood cells suspended in the tissue culture medium as perfusate(5, 6), in this study 1) maternal perfusates consisted of autologous maternal blood obtained from intervillous space and diluted with modified TC-199 medium(15) and 2) fetal perfusate comprised autologous cord blood diluted with TC-199 medium. Hence, we did not add bovine albumin to the perfusate because the endogenous concentration of albumin was high enough to maintain colloid-osmotic pressure. Closed circuit perfusion of the fetoplacental circulations was established by cannulating the chorionic artery and vein. Fetal perfusion pressure and venous outflow was monitored continuously throughout the experiments by an on-line pressure transducer and flow probe. The fetal perfusion pressure was 24-30 mm Hg with a venous outflow of 8-10 mL min-1. Closed circuit maternoplacental circulation was established by placing five cannulas in the intervillous space with an arterial pressure of 10-12 mm Hg and a flow rate of 20-24 mL min-1. Maternal and fetal circulating volumes were 150 and 120 mL, respectively. Maternal and fetal perfusates had mean hematocrit values of 7.5 (range 4-9) and 21 (range 16-25), respectively. Maternal and fetal hematocrits in this study differed from those reported previously(5, 6), where washed maternal and fetal blood cells suspended in the tissue culture medium were used as the perfusate. Initially during establishment of perfusion, both maternal and fetal circuits were oxygenated. Thereafter, tissue oxygenation was maintained by oxygenating the maternal circulation with 95% oxygen and 5% carbon dioxide. Perfusion efficiency and diffusional transfer rate was determined in each experiment by measuring the rate of transplacental transfer of a freely diffusible marker, creatinine(Mr 113). Experiments were discarded if 1) maternal to fetal transfer of creatinine was not linear or greater than predefined range of 8-16% of the initial dose(16), 2) if fetal perfusion pressure increased by >10 mm Hg, and 3) fetal circulating volume dropped by >2-3 mL. Thirty-three placentas were perfused. Five experiments were discarded because transplacental transfer of creatinine was outside the predefined range.
In five experiments, 750 μIU of TSH, with creatinine (30 mg) were added as a single bolus to the closed maternal circulation to attain an estimated drug concentrations of 5 μIU mL-1 and 0.2 mg mL-1, respectively. This concentration of TSH (5 μIU mL-1) was selected to mimic endogenous maternal basal plasma concentration(7, 17) and secondarily to ensure that fetal levels would fall within the assay sensitivity.
To determine the effect of maternal administration of TSH on the rate of placental transport, perfusion experiments were also undertaken in which 1500(n = 4) or 3000 μIU (n = 6) of TSH were added to the maternal circulation to attain estimated drug concentrations of 10 and 20μIU mL-1, respectively. These concentrations were chosen because they correspond to the expected range of maternal levels of TSH achieved after 400 μg of TRH i.v.(17). In addition, we also studied the transfer of TSH by adding supraphysiologic quantities of 6000 μIU (40μIU mL-1; n = 6) and 9000 μIU (60 μIU mL-1;n = 7) of TSH to the maternal circulation. Maternal concentrations of TSH were calculated based on the maternal circulating volume of 150 mL and were confirmed in maternal samples collected at 6 min. The differences between estimated and measured levels were <3-5%.
Two milliliters of maternal and fetal samples were collected at 15-min intervals over 2 h and replaced with fresh perfusate. At the end of the perfusion period, both circuits were drained, and their volumes were measured. The perfused lobule was then excised and pressure blotted to remove the perfusate from the intervillous space. Samples were centrifuged at 3000× g for 15 min, and plasma was stored at -20°C for subsequent analysis.
Placental uptake of TSH was measured by homogenizing the perfused placental tissue in an Ultraturrax high speed homogenizer in 380-420 mL of PBS. Ten-milliliter aliquots of the homogenized tissue were centrifuged at 3000× g for 15 min.
Maternal and fetal concentrations of TSH were expressed as percentages of the initial dose added to the maternal circulation after correction for background activity, circuit volume, and the amount removed from previous samplings. Placental tissue concentrations were expressed as percentages of the initial dose added to the maternal circulation.
Membrane transfer. Intact human fetal membranes comprising amnion, chorion, and decidua were obtained from normal pregnancies between 37 and 42 wk of gestation immediately after vaginal or cesarean delivery. Membranes were washed in PBS to remove blood, were mounted over the end of a 20 mm × 16-mm wide hollow glass cylinder, and held in place with silicon rubber O rings as described previously(8, 18). Excess membranes were trimmed. The experimental preparations were placed on a 12-well tissue culture plate in such a manner that membranes just dipped into the culture medium, but did not touch the base of the wells. Culture medium(1.5 mL) was comprised of TC-199 with 10% ITS (insulin, 12.5 mg; transferrin, 12.5 mg; selenium, 12.6 μL; linoleic acid, 11.1 μL; and BSA, 2.5 g in 20 mL), 2 mmol of glutamine, and 1% penicillin-streptomycin. A 1.5-mL aliquot of this culture medium was added to each side of the membrane to produce a two-compartmental experimental system. In all experiments, choriodecidua(maternal side) faced downward toward the base of the well of the culture plate. After overnight incubation of the membranes at 37°C in 95% O2 and 5% CO2, the tissue culture medium was replaced with fresh medium.
Then 45 μIU of TSH, 25 μg of creatinine, and 30 μg of antipyrine were added either to the maternal or fetal compartments of the membranes obtained from seven separate placentas. The membranes were then incubated for 2-8 h. Time-dependent transfer across the membrane was determined at 0.5, 1, 2, 4, 6, and 8 h of incubation. At each time point membranes were incubated in triplicate. A control was included at each point to determine the endogenous production of TSH. Heparin (650 IU) was used as a within experimental control to ascertain diffusional viability of the preparations, and experiments where transfer of heparin was >1.5% at 8 h were discarded. Creatinine and antipyrine were also used to compare the transfer across the membrane with that of the placenta. The cellular viability of the membrane at each time point was determined by trypan blue and only those experiments where the membrane disc showed no blue staining were considered valid(18). One experiment was discarded because transfer of heparin was found to be >10% at several time points because of disruption of the membrane.
Analytical methods. TSH concentration was measured by a highly specific third generation chemiluminescence assay with a coefficient of variation of 5-10% and sensitivity of 10 mIU mL-1. The creatinine concentration was determined by colorimetric assay(19), with a coefficient of variation of 7-12%. Antipyrine levels were measured spectrophotometrically(20) with a coefficient of variation of 10-14%. Heparin levels were estimated colorimetrically(21) with a coefficient of variation of 10-14% and lower limit of detection of 0.1 IU mL-1.
Data analysis. All values were expressed as mean ± SEM unless otherwise indicated. Data between two groups as a function of time were compared by two-way ANOVA. Oneway ANOVA was used to compare blocked data between groups. p values <0.05 were considered significant. Equilibrium between maternal and fetal circuits was achieved when fetal/maternal ratios of the drug levels approached unity. The area under the curve of maternal (MAUC) and fetal (FAUC) concentration of a drug was calculated by using the trapezoidal rule(13). The placental transfer rate of TSH was determined by fitting a weighted line for the standard deviations of means of the fetal concentration of TSH between 15 and 120 min and calculating its slope.
Placental and fetal membrane permeability of TSH, heparin, and creatinine were calculated by the equation(13): where PS = placental permeability in mL min-1 g-1 of perfused placental tissue, n = total fetal plasma concentration at 120 min (Cm - Cf)dt = integrated concentration differences across the placenta and membranes, calculated by subtracting MAUC from FAUC.

Molecular size of TSH was calculated by the Einstein-Stokes formula. The coefficients of free diffusion in water of TSH, heparin, and creatinine at 37°C were calculated from their molecular weights: D = 5.1·10-5/(molecular weight0.40) × 0.649(13). The permeability of fetal membrane to antipyrine was not determined because it is lipid soluble and transfer across the membrane depends upon the flow rate.
RESULTS
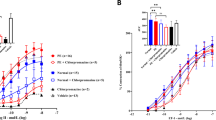
Transfer across the placenta. When 5 μIU mL-1 TSH was added to the closed maternal circulation, maternal levels decreased from 100% at 0 min to 96.4 ± 0.8% at 120 min, as shown in Figure 1. The fetal concentration increased linearly from undetectable levels at 0 min, to 0.11 ± 0.04% at 120 min. The transfer rate of TSH was 0.08 ± 0.002 μIU·min-1 with a permeability coefficient of 2.4 ± 0.2·10-4 mL min-1 g-1. The MAUC and FAUC values of TSH were 516 ± 15μIU min-1 mL-1 and 0.7 ± 0.2 μIU min-1 mL-1, respectively. The F/M and FAUC to MAUC ratio of TSH at 2 h was 0.002 ± 0.0004 and 0.001 ± 0.0003, respectively. The placental uptake of TSH was 0.04 ± 0.01% of the maternal dose. The total recovery of TSH was 98.7 ± 1.6% dose.
Maternofetal transfer of TSH when added as a single bolus dose of (A) 5 μIU mL-1 (n = 5);(B) 10 μIU mL-1 (n = 4); (C) 20 μIU mL-1 (n = 6); (D) 40 μIU mL-1(n = 6); and (E) 60 μIU mL-1 (n = 7) to the maternal circulation at the commencement of the experiments. Maternal(•–•) and fetal (○–○) concentrations are expressed as percent of initial dose added. In these experiments both maternal and fetal perfusates were recirculated.
When maternal concentration of TSH was increased from 5 μIU mL-1 to 10 μIU mL-1, 20 μIU mL-1, 40 μIU mL-1 and then a maximum of 60 μIU mL-1, there was a proportional increase in fetal levels (y = 0.007x; R2 = 0.98), placental uptake (y = 0.01x; R2= 0.97) and transfer rate(y = 0.002x; R2 = 0.99) of TSH. Placental uptake of TSH was less than the percent dose of TSH transferred at 120 min (y= 0.56x; R2 = 0.87; p < 0.001)(Fig. 2). FAUC (y = 0.031x; R2= 0.99; p < 0.001), F/M ratio (y = 0.001x; R2 = 0.99; p < 0.001), and FAUC/MAUC ratio (y= 0.001x; r = 0.99; p < 0.001) of TSH increased with increasing maternal concentration (Fig. 3).
Relationship between the maternal concentration of TSH and (A) fetal concentration at 120 min (y = 0.007x; R2 = 0.98; p < 0.001; n = 28); (B) transfer rate (y = 0.002x; R2= 0.99; p< 0.001; n = 28); (C) placental uptake (y = 0.01x; R2 = 0.98; p < 0.01; n = 28);(D) correlation between fetal concentration and placental uptake of TSH (y = 0.56x; R2 = 0.88; p < 0.001;n = 28).
Transfer across the membrane. Transfer across the fetal membranes was studied in both maternal to fetal and fetal to maternal directions. The net transfer of TSH, antipyrine, creatinine, and heparin from maternal to fetal and fetal to maternal sides was comparable.
Maternal levels of TSH decreased from 100% at 0 min to 87.2 ± 3.2% at 8 h with a concomitant linear albeit minimal increase in fetal levels from undetectable levels at 0 min, to 4.0 ± 0.5% at 8 h (r = 0.91; slope of 0.001) (Fig. 4). The transfer rate of TSH was 0.06 ± 0.002 μIU min-1 with a permeability coefficient of 3.0± 0.1 mL min-1 g-1. The MAUC and FAUC values of TSH were 305 ± 6 μIU min mL-1 and 8.2 ± 0.7 μIU min mL-1, respectively. The F/M and FAUC to MAUC ratio of TSH was 0.05± 0.004 and 0.03 ± 0.002, respectively. The total recovery of TSH was 97 ± 2% per dose. The transfer of heparin in these experiments was 1.0 ± 0.1% with a permeability coefficient and transfer rate of 9.0± 0.7·10-5 mL min-1 g-1 and 0.004 ± 0.001 IU min-1, respectively.
Transmembrane transfer of TSH from (A) maternal (•–•) to fetal (○–○) and (B) fetal(○–○) to maternal (•–•) compartment. Fetal concentration of heparin is shown as interrupted line (δ–δ).(C) Transfer of creatinine (△) and antipyrine(□) from maternal to fetal compartment, and (D) from fetal to maternal compartment. Closed and open symbols represent maternal and fetal drug concentration, respectively. Values were obtained from seven experiments and expressed as percent of initial dose added.
The transmembrane transfer of antipyrine was significantly greater than that of creatinine (32.9 ± 0.9 versus 18.7 ± 1.5;p < 0.01) (Fig. 4,B and D). Similarly, the transfer rate of antipyrine, 1.2 ± 0.1 mg min-1, was higher than that of creatinine, 0.6 ± 0.001 mg min-1, as was the F/M ratio(0.54 ± 0.02 versus 0.3 ± 0.03; p < 0.001) and TSH (p < 0.001) (Fig. 5). The permeability of membrane to creatinine was 0.02 mL min-1 g-1.
DISCUSSION
We studied the placental permeability to TSH in relation to varying maternal concentrations in an in vitro model of perfused human term placenta and found that placental transfer of TSH is dose-dependent. The low fetal levels found at high maternal concentrations are unlikely to be of clinical significance, and these findings support the clinical observation that the human placenta is relatively impermeable to maternal TSH(9).
Previously we have used the perfused placental model to determine the permeability of macromolecules and obtained results that corroborate those from in vivo studies(15, 22). Because the half-life of TSH in vivo is estimated to be 60 min, the 2-h duration of perfusion experiments was considered sufficient to evaluate transplacental pharmacokinetics.
The placental clearance of TSH was minimal despite flow rates into the perfused lobule of 24-26 mL min-1 on the maternal and 8-10 mL min-1 on the fetal side. Shunting of blood flows within the perfused lobule is unlikely to be the cause of poor transfer of TSH because in each experiment transfer of freely diffusible molecules was similar to that reported by other investigators(15, 16, 23). Instead, it appears that placenta forms an anatomical barrier to passive diffusion of TSH. Low molecular weight compounds such as aspirin, warfarin, phenytoin, and diazepam(22, 24) are all known to cross the placenta readily. Under similar experimental conditions we and others have previously shown that substances with molecular weight>1000 such as inulin, human serum albumin, and bovine IgG do not cross the placenta readily, and the rate of placental transfer is proportional to the molecular weight(23, 25, 26). Hence the poor transplacental passage of TSH despite increasing maternal concentrations could be due to its relatively high molecular weight of 28 000.
Degradation of TSH by the placenta is another possibility. TSH is a glycoprotein consisting of α and β subunits. The β unit confers functional specificity and is involved in binding of TSH to the thyroid cell membrane. Clearance of TSH from the circulation occurs predominantly by enzymatic degradation of the β subunit in the kidney(27). Although we made no attempt to determine individual metabolites of TSH, the total recovery of 95-97% of TSH as determined by a highly specific assay for β subunits, in all perfusion experiments, precludes degradation of TSH as a possible mechanism for failure of transfer across the placenta. Placental degradation of TSH into componentα and β subunits is another possibility. The biologic relevance of this remains indeterminate more so when evidence suggests that for maximal physiologic activity both units of TSH must be linked together.
A further explanation could be that TSH is transferred by an energy-dependent pathway. Although no direct information is available regarding the mechanism by which TSH crosses the placenta, we speculate that, like most drugs, transport occurs by simple diffusion. The observation of linear relationship between the rate of transport and the concentration of TSH is consistent with transfer by passive diffusion. Had it been by an energy-dependent pathway, a saturable process for the transport and uptake of TSH with an increasing concentration would be expected. A positive correlation of maternal concentration with placental uptake and with fetal AUC is consistent with a first order passive diffusion process. Furthermore, the rate of transfer of TSH was at least 3-fold greater than the placental uptake, suggesting that placental transport of TSH occurs by paracellular passive diffusion.
Morphologic evidence indicates that resistance to free placental transfer of a hydrophilic molecules like TSH is likely to be in the trophoblastic layer that forms a continuous syncytium in the human placenta(28). However, physiologic data indicate that the transfer of low molecular mass hydrophilic substances such as urea (60 D), creatinine (113 D), and erythritol (122 D) occurs at a much slower rate than lipid soluble molecules of similar size(29, 30). This differential rate is largely because transfer of hydrophilic substances depends on placental permeability but is independent of placental blood flow or protein binding. Accordingly, transfer of hydrophilic substances such as inulin (7%), chlorazepate (20%), heparin (1.2%), and cimetidine (23%) are found to be lower than the flow-dependent marker antipyrine(15, 26, 30, 31). The placental clearance of TSH of 1.5% of antipyrine further suggests that maternal to fetal transfer depends on placental permeability.
There is now considerable circumstantial and experimental evidence that the human, unlike the sheep, placenta is permeable to hydrophilic substances, and transfer occurs through water-filled paracellular pathways of >17 Å size(15, 11, 32),(33). The measured permeability of TSH in this study was comparable to that estimated from interpolation of published data on the permeability of other hydrophilic molecules, providing further evidence of the presence of paracellular pathways of pore size greater than the molecular size of TSH (26 Å). Our finding that the permeability of TSH, despite its larger molecular size, is greater than that reported for heparin(15), supports Bain et al.'s(34) suggestion that transfer of macromolecules across the human placenta depends upon their polarity.
We also studied the transfer of TSH in an in vitro dual chamber model of fetal membrane in culture to investigate an alternative pathway by which TSH might enter into the fetal compartment. Although this system has not been used extensively to study drugs or hormone transfer across chorioamniotic membranes, various investigators using a similar system have shown endogenous production of interleukins, prostaglandins, and prolactin(34–37). The culture conditions to preserve membranous cellular integrity and suitability to study transfer of drugs have been optimized in our laboratory(8, 38, 39).
Using this system, we found that TSH crosses the fetal membranes poorly. In contrast, inert low molecular weight substances cross the membrane freely, indicating that permeability of the fetal membrane to drugs depends upon their molecular weight. However, our observation that transmembrane transfer of TSH was significantly higher than for polar molecules like heparin indicates that factors other than molecular weight may influence transmembrane passage of inert molecules. In keeping with this, we also found that, despite similar molecular weight, transmembrane transfer of a lipophilic inert substance like antipyrine (molecular mass = 130 D) was markedly higher than a hydrophilic compound like creatinine (molecular mass = 113 D). These observations, therefore, suggest that, like placenta, membrane permeability also depends upon the polarity and lipid solubility of a drug. Although the clinical significance of transmembrane transfer remains unclear, it is possible that drugs or nutrients may also gain access to the fetal compartment by a route other than the placenta.
Assuming that transmembrane transfer of TSH, heparin, and creatinine occurs by simple diffusion, we calculated the permeability of amnion and chorion to these hydrophilic molecules and found that, like placenta, they were inversely related to molecular size. The observation of minimal transfer across the membranes suggests that amniotic fluid TSH is unlikely to be of maternal origin.
In conclusion, our data suggest that TSH does not cross the human placenta or fetal membranes in significant quantities and indicate that the TRH-stimulated peak TSH response in the mother is unlikely to elicit a fetal thyrotrophic response. Given the recent finding that the human term placenta forms an enzymatic barrier to free passage of TRH(5–8), the mechanism by which maternal TRH administration in vivo leads to a fetal TSH response remains unclear. Placental release of TSH-like substances is one possibility. Further studies are needed to elucidate the precise mechanism for the fetal thyrotrophic response to maternal TRH administration.
Abbreviations
- MAUC:
-
maternal area under the curve
- FAUC:
-
fetal area under the curve
- F/M:
-
fetomaternal
- TRH:
-
TSH-releasing hormone
References
Crowley P, Chalmers I, Keirse MJ 1990 The effects of corticosteroid administration before preterm delivery: An overview of the evidence from controlled trials. Br J Obstet Gynaecol 97: 11–25.
Roti E, Gnudi A, Braverman LE 1993 The placental transport, synthesis and metabolism of hormones and drugs which affect thyroid function. Endocr Rev 4: 131–149.
Ballard PL, Ballard RA, Creasy RK, Padbury J, Polk DH, Bracken M, Moya FR, Gross I 1992 Plasma thyroid hormones and prolactin in premature infants and their mothers after prenatal treatment with thyrotropin-releasing hormone. Pediatr Res 32: 673–678.
Crowther CA, Alfirevic Z 1995 Antenatal thyrotropin-releasing hormone prior to preterm delivery. In: Keirse MJMC, Renfrew MJ, Crowther CA, Neilson JP (eds) Cochrane Database of Systematic Reviews-Pregnancy and Childbirth Module, Version 1.2, Disk Issue 2, Record 4749
Bajoria R, Oteng-Natim E, Fisk NM 1996 Transfer and metabolism of thyrotropin releasing hormone across the perfused human term placenta. J Clin Endocrinol Metab 81: 3476–3482.
Bajoria R, Fisk NM 1997 Maternal-fetal transfer of thyrotropin releasing hormone: effect of maternal dose and concentration. Pediatr Res 41: 674–681.
Bajoria R, Peek M, Fisk NM Maternal to fetal transfer of thyrotrophin releasing hormone in vivo. Am J Obstet Gynaecol, in press
Bajoria R, Ryder TA, Fisk NM 1997 Transport and metabolism of thyrotrophin releasing hormone across the fetal membrane. J Clin Endocrinol Metab 82: 3399–3407.
Heinrichs C, de-Zegher F, Vansnick F, Vokaer A, Christophe C, Frankenne F 1994 Fetal hypopituitarism: perinatal endocrine and morphological studies in two cases. Acta Paediatr 83: 448–451.
Yoshida K, Sakurada T, Takahashi T, Furuhashi N, Kaise K, Yoshinaga K 1986 Measurement of TSH in human amniotic fluid: diagnosis of fetal thyroid abnormality in utero. Clin Endocrinol 25: 313–318.
Robuschi G, Braverman LE, Emanuele R, d'Amato L, Gardini E, Foscolo MS, Gualerzi C, Benassi L, Gnudi A, Roti E 1985 Amniotic fluid thyrotropin (TSH) following maternal administration of thyrotropin releasing hormone. J Perinat Med 13: 219–226.
Hollingsworth DR, Alexander NM 1983 Amniotic fluid concentrations of iodothyronines and thyrotropin do not reliably predict fetal thyroid status in pregnancies complicated by maternal thyroid disorders or anencephaly. J Clin Endocrinol Metab 57: 349–355.
Bain MD, Copas DK, Landon MJ, Stacey TE 1988 In vivo permeability of the human placenta to inulin and mannitol. J Physiol 399: 313–319.
Thornburg KL, Burry KJ, Adams AK, Kirk EP, Faber JJ 1988 Permeability of placenta to inulin. Am J Obstet Gynecol 158: 1165–1169.
Bajoria R, Contractor SF 1992 Transfer of heparin across the human perfused placental lobule. J Pharm Pharmacol 44: 952–959.
Eaton BM, Browne MJ, Contractor SF 1985 Maternal to fetal movement of creatinine as a measure of perfusion efficiency and diffusional transfer in the isolated human placental lobule. Placenta 6: 341–346.
Bajoria R, Oteng-Natim E, Peek M, Fisk NM 1997 Pharmacokinetics and pharmacodynamics of TRH during pregnancy. Obstet Gynaecol 90: 187–192.
Roseblade CK, Sullivan MH, Khan H, Lumb MR, Elder MG 1990 Limited transfer of prostaglandin E2 across the fetal membrane before and after labor. Acta Obstet Gynecol Scand 69: 399–403.
Heinegard D, Tiderstrom G 1973 Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta 43: 305–310.
Brodie BB, Axelrod J, Soberman R, Levy BB 1949 The estimation of antipyrine in biological materials. J Biol Chem 179: 25–31.
Klein MD, Drongowski RA, Linhardt RJ, Langer RS 1982 A colorimetric assay for chemical heparin in plasma. Anal Biochem 124: 59–64.
Bajoria R, Contractor SF 1993 Maternal-fetal transfer of warfarin across an in vitro model of perfused placenta; effect of albumin binding. Br J haematol 84: 59
Contractor SF, Eaton BM, Stannard PJ 1983 Uptake and fate of exogenous immunoglobulin G in the perfused human placenta. J Reprod Immunol 5: 265–273.
Reynolds F, Knott C 1989 Pharmacokinetics in pregnancy and placental drug transfer. Oxf Rev Reprod Biol 11: 389–449.
Dancis J, Jansen V, Levitz M 1976 Transfer across perfused human placenta. IV. Effect of protein binding on free fatty acids. Pediatr Res 10: 5–10.
Contractor SF, Stannard PJ 1983 The use of AIB transport to assess the suitability of a system of human placental perfusion for drug transport studies. Placenta 4: 19–30.
Ridgway EC, Weintraub BD, Maloof F 1974 Metabolic clearance and production rates of human thyrotropin. J Clin Invest 53: 895–903.
Edwards D, Jones CJ, Sibley CP, Nelson DM 1993 Paracellular permeability pathways in the human placenta: a quantitative and morphological study of maternal-fetal transfer of horseradish peroxidase. Placenta 14: 63–73.
Illsley NP, Hall S, Penfold P, Stacey TE 1985 Diffusional permeability of the human placenta. Contr Gynecol Obstet 13: 92–97.
Schneider H, Sodha RJ, Progler M, Young MPA 1985 Permeability of the human placenta for hydrophilic substances studied in the isolated dually in vitro perfused lobe. Contr Gynecol Obstet 13: 98–103.
Ching MS, Mihaly GW, Morgan DJ, Date NM, Hardy KJ, Smallwood RA 1987 Low clearance of cimetidine across the human placenta. J Pharmacol Exp Ther 241: 1006–1009.
Willis DM, O'Grady JP, Faber JJ, Thornburg KL 1986 Diffusion permeability of cyanocobalamin in human placenta. Am J Physiol 250:R459–R464.
Stulc J 1988 Is there control of solute transport at placental level. Placenta 9: 19–26.
Bain MD, Copas DK, Taylor A, Landon MJ, Stacey TE 1990 Permeability of the human placenta in vivo to four non-metabolized hydrophilic molecules. J Physiol 431: 505–513.
McCoshen JA, Barc J 1985 Prolactin bioactivity following decidual synthesis and transport by amniochorion. Am J Obstet Gynecol 153: 217–223.
Kent AS, Sullivan MH, Sun MY, Zosmer A, Elder MG 1993 Effects of interleukin-6 and tumor necrosis factor-alpha on prostaglandin production by cultured human fetal membranes. Prostaglandins 46: 351–359.
Fortunato SJ, Menon RP, Swan KF, Menon R 1996 Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-α) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol 174: 1855–18561.
Kent AS, Sullivan MH, Elder MG 1994 Transfer of cytokines through human fetal membranes. J Reprod Fertil 100: 81–84.
Morris C, Sullivan MH, Elder MG 1992 Transfer and metabolism of platelet-activating factor by fetal membranes, amnion and chorio-decidua. Br J Obstet Gynaecol 99: 895–898.
Author information
Authors and Affiliations
Additional information
Supported by a project grant from Action Research.
Rights and permissions
About this article
Cite this article
Bajoria, R., Fisk, N. Permeability of Human Placenta and Fetal Membranes to Thyrotropin-Stimulating Hormone in Vitro. Pediatr Res 43, 621–628 (1998). https://doi.org/10.1203/00006450-199805000-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199805000-00010
This article is cited by
-
Fetal Membrane Transport Enhancement Using Ultrasound for Drug Delivery and Noninvasive Detection
Pharmaceutical Research (2015)