Abstract
Cerebral magnetic resonance imaging (MRI) has revealed white matter changes in patients with phenylketonuria (PKU), an inborn error of metabolism with increased plasma phenylalanine level. Because the significance of these lesions is unknown, this study was undertaken to determine whether glucose metabolism was depressed in cerebral white matter MRI changes in patients with PKU. Four patients with PKU and nine healthy volunteers with an average age of 23 y (range 19-26 y) and 23 y (range 20-27 y), respectively, were studied. The IQ of patients with PKU was between 58 and 97. Cerebral MRI and positron emission tomography images with 18F-deoxyglucose were obtained, and arteriovenous differences for oxygen and glucose as well as cerebral blood flow was measured simultaneously to determine global cerebral oxygen and glucose metabolism. Cerebral MRI revealed that all patients with PKU had white matter changes with characteristic localization. In patients with PKU, regional glucose metabolism was 36% lower in the anterior periventricular areas, 0.14 ± 0.06 compared with 0.22 ± 0.04μmol·g-1·min-1 in controls (mean ± SD,p < 0.05, Mann-Whitney). Further, the ratio between glucose metabolism in the affected white matter and the cortex was 14% lower in the patients, decreasing from 0.57 ± 0.05 to 0.48 ± 0.06(p < 0.05). Global cerebral blood flow, oxygen and glucose consumption were similar in the two groups. In conclusion, regional glucose metabolism is lower in MRI-demonstrated white matter changes. In mildly intellectually impaired patients with PKU, global cerebral glucose and oxygen metabolism remain intact.
Similar content being viewed by others
Main
Untreated PKU is an invalidating inborn error of metabolism which is inherited in an autosomal recessive manner. Early and sustained treatment with phenylalanine-restricted diet is mandatory to avoid long-term cerebral damages with mental retardation, autistic behavior, and seizures.
Cerebral MRI has revealed profound white matter changes in adults with PKU and low intelligence or severe neurologic sequelae(1, 2), but these changes may also be present in patients with controlled hyperphenylalaninaemia(3, 4). It is well established that the white matter changes preferentially are localized to the occipital lobes close to the posterior part of the ventricles, but the pathogenesis of the increased signal intensity is unknown. Proton spectroscopy has revealed a normal spectrum, indicating that the lesions are not associated with severe neuronal damage or ongoing myelin degradation(3, 5, 6). In optimally treated PKU, however, the changes seem to be functionally associated with a subtle complex pattern of perception defects, and occipital EEG abnormalities are often seen(3). The present study was undertaken to determine whether glucose metabolism disturbances are present in regions with white matter changes.
METHODS
Patients. Four male patients with PKU and eight male and one female healthy volunteers were studied. Average age of the patients was 23 y(range 19-26 y), and of the volunteers it was 23 y (range 20-27 y). Three of the four patients had at the age of 5 y a phenylalanine tolerance between 16 and 20 mg/kg (classical PKU), whereas the last patient had a phenylalanine tolerance of 28 mg/kg (moderate PKU). The IQ of patients with classical PKU was between 58 and 69 (WAIS full scale IQ), and they all exhibited slight to moderate EEG abnormalities. Two of the patients received disablement pension. The patient with moderate PKU had an IQ of 97 (WISC), and EEG examination was normal. Apart from mild tremor and increased tendon reflexes, neurologic examinations were unremarkable. All four patients had been treated with phenylalanine-restricted diet since they were between 2 and 16 wk old and until the age of 15 y. Thereafter, the diet was liberated. For the preceding 2-3 y, the patients were treated with Tyrotryp-S tablets (Jensen Clinical Nutrition, Dianalund, Denmark) containing 256 mg of L-tyrosine and L-tryptophan and 244 mg of other essential amino acids(7). The participants also entered a study on blood-brain barrier permeability to amino acids, which has been published previously(16). The study was approved by the committee for Ethics in the Copenhagen and Frederiksberg Area. Written informed consent was obtained from each participant.
Cerebral arteriovenous differences. Catheters were placed in the radial artery, in the internal jugular vein, and in an antecubital vein in all patients and in four volunteers. Arteriovenous differences for oxygen and glucose were measured simultaneously from the radial artery and the internal jugular vein. Plasma glucose was measured with a Beckmann glucose analyzer(Beckmann Instruments, Inc., Fullerton, CA). Arteriovenous differences for glucose was measured five times during the study, and mean values were used. Global cerebral oxygen and glucose consumption were calculated by multiplying CBF with the corresponding arteriovenous differences.
Measurement of CBF. Cerebral blood flow was measured in each study using the 133Xe i.v. injection method, and washout from the brain was monitored by a mobile 10 detector unit (Cerebrograph 10A, Hadsund, Denmark)(8).
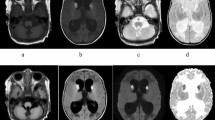
MRI scanning. MRI was performed at 1.5 T (Siemens Magnetom SP 63/84). Axial T2-weighted images were obtained by double spin-echo sequence (TR = 2.5 s, TE = 15 and 90 ms). The slice position was tilted to parallel the canthomeatal plane.
PET-FDG scanning. The subject was placed supine with eyes closed and ears unplugged in quiet dim surroundings. PET scanning was performed with a Therascan 3128 PET scanner (Atomic Energy Ltd, Canada) from 45 to 90 min after injection. An i.v. injection of 180-250 MBq FDG were given, and simultaneously, 1.5-mL blood samples were drawn from the radial artery with increasing time intervals and immediately placed on ice. The samples were centrifuged, and plasma activity was counted in a gamma counter (COBRA 5003, Packard Instruments, Downers Grove, IL). Correction for deadtime, random scatter, and attenuation was performed as previously described(9). Regional cerebral glucose metabolism was measured by the autoradiographic approach, first described by Sokoloff et al.(10) and later modified by Brooks(11). Fixed rate constant values were used:k1 = 0.105, k2 = 0.126, k3= 0.075, and k4 = 0.0068. The lumped constant was fixed at 0.56. Several plasma glucose measurements were performed during the study with a Beckman glucose analyzer (Beckman Instruments Inc.), and values were averaged.
Data analysis. A common positioning technique and an atlas-derived ROI definition was used to identify brain structures on the MRI scans and the corresponding functional areas on the PET-FDG images. The canthomeatal plane was used as a reference for both MRI and PET-FDG scans, and the midplanes of the nine slices were placed at the same levels above the canthomeatal plane for both studies. Because slice thickness was approximately the same (MRI: 10 mm, PET: 12 mm), the volume of the brain covered by these scans was comparable. With reference to an anatomic atlas(12), nine brain levels, parallel to the canthomeatal plane separated by 10 mm, were selected. In each of these levels, a template of symmetric cortical and subcortical ROI was defined, as previously described in detail(13). For each level, the ROI template was drawn on the MRI slice and then applied to the PET image, using a VAX-Station. It was allowed only to redraw or adjust the outer boundaries of the brain, because these could be influenced by minute positioning errors between MRI and PET. The ROI were incorporated into larger regions of meaningful anatomical localization, which could comprise several slices(13). The weighted mean metabolism value of the larger ROI was calculated from the mean pixel value and the area of the region for each slice in which the ROI appeared. ROI with white matter changes defined from MRI were applied similarly to the PET images, and intraindividual ratios between CMRglc in white matter changes and mean total cortical CMRglc were calculated.
Statistics. Mann-Whitney test was used for comparison of CBF, global cerebral oxygen, and glucose consumption, and regional glucose metabolism between the two groups. A significance level of 0.05 was chosen.
RESULTS
Plasma phenylalanine levels were about 20 times higher in the patients, mean ± SD values were 1.12 ± 0.45 mM in patients and 0.050± 0.002 mM in the controls (n = 4). Cerebral MRI revealed that all patients with PKU had white matter changes both frontally and in the occipital lobes. None of the patients had enlargement of ventricles. All volunteers had normal MRI images.
Values for CBF, cerebral oxygen metabolism, and the ratio between glucose consumption in white matter changes and cortex are shown inTable 1. Figure 1 shows global and regional glucose metabolism for selected regions. Global CBF, oxygen, and glucose consumption was similar in the two groups. In patients with PKU, regional glucose metabolism was significantly decreased by 36% in the anterior periventricular areas, and the ratio between glucose metabolism in the white matter changes and cortical regions decreased by 14%. In the other selected brain regions, there was no difference between patients and controls.
Global and regional glucose metabolism in patients with PKU compared with healthy controls. GL, global cerebral glucose metabolism; FC, frontal cortex; TC, temporal cortex;PC, parietal cortex; OC, occiptal cortex; SC, semiovale center; PA, periventricular anterior area; PO, periventricular occipital area. Values for glucose metabolism areμmol·g-1·min-1, mean ± SD.*p < 0.05.
DISCUSSION
In all patients with PKU, MRI revealed white matter hyperintensity changes localized to areas typically affected in PKU, i.e. adjacent to the occipital horns. The nature and pathophysiology of these changes still remain unknown. Although the MRI abnormalities are primarily located to white matter brain regions, there seems, however, to be little doubt that gray matter regions are functionally affected, because both intellectual impairment, neuropsychologic disturbances, and EEG abnormalities are frequently seen.
In patients with PKU, white matter glucose metabolism was reduced in the areas of MRI changes localized preferentially in the posterior periventricular areas, as revealed by a decrease in the white matter changes/cortex ratio. The finding of a relative reduction in glucose metabolism in these areas indicates that, whatever causes the MRI signal hyperintensities, they are associated with a decrease in metabolic demand and thus function. Glucose metabolism was also lower in anterior periventricular areas outside of MRI changes which render probable that glucose metabolism is reduced not only in areas of MRI white matter changes but also in surrounding white matter. It was somewhat surprising that absolute glucose consumption in the posterior periventricular white matter seemed normal. This may be caused by difficulties in selection of“pure” white matter regions on PET images, because the posterior periventricular white matter region is characterized by deep gyri with high metabolic activity, which, due to the spatial resolution of the images, tends to overestimate white matter metabolism in these regions. In the other selected brain regions there was no difference between patients and controls.
It could be argued that our finding of normal glucose metabolism in cortical tissue in patients with PKU may be caused by a combination of the statistical scatter in glucose consumption determination and the limited sample size. Absolute values for glucose metabolism measured with PET-FDG are obtained with a considerable intersubject variability due to methodologic problems(14), whereas internal ratios are less variable. The ratio between glucose metabolism in the white matter changes and cortical regions is therefore a relatively solid parameter, which decreased significantly in patients with PKU and which signifies that, if anything, depression of cortical glucose metabolism is less pronounced even in the presence of moderate intellectual impairment. A normal cortical glucose metabolism could also be explained by the primary pathology being located subcortically with subsequent deafferentation of cortical tissue overlying the white matter changes. It is possible that undercutting may preserve or even enhance cortical glucose metabolism, in line with the notion that glucose consumption and task performance are negatively correlated in normal adults and that learning induces a reduction in glucose metabolism(15).
Global cerebral blood flow and oxygen and glucose consumption were similar in the two groups, signifying that, even in moderately mentally retarded patients, changes in brain flow and metabolism are subtle and heterogenous. It is not too surprising that the changes are relatively small, because a large part of brain oxygen and glucose consumption is used for maintenance of ion gradients, and the number of neurons seems to be relatively preserved in PKU as indicated by a normal N-acetyl-aspartate/creatine ratio found by MRI spectroscopy(3, 5, 6). Although it may be difficult to make firm conclusions based on this relatively new method, taken together with the present data they indicate that no severely abnormal global brain metabolism is found in these patients.
We conclude that glucose metabolism is decreased in white matter with increased T2 in patients with PKU, suggesting that the MRI changes found in this disorder is not a trivial epiphenomenon. Other modalities have so far failed to disclose any significant abnormality specifically located in white matter changes as opposed to other brain areas. A relative increase in white matter inositol concentration has been found with MRI spectroscopy in PKU, but there was no correlation between inositol concentration and MRI pathology(5). It is possible that functional imaging with MRI or PET using blood flow tracers or FDG, would reveal further disturbances in glucose metabolism or blood flow during activation tasks, e.g. pattern recognition tests. Future research should be directed toward elucidation of possible functional deficits which may improve our understanding of specific difficulties experienced by patients with PKU or other types of inborn errors of metabolism.
Abbreviations
- CBF:
-
cerebral blood flow
- CMRglc:
-
cerebral metabolic rate for glucose
- MRI:
-
magnetic resonance imaging
- PET:
-
positron emission tomography
- PKU:
-
phenylketonuria
- ROI:
-
region of interest
References
Thompson AJ, Smith I, Brenton D, Youl BD, Rylance G, Davidson DC, Kendall B, Lees JA 1990 Neurological deterioration in young adults with phenylketonuria. Lancet 336: 602–605
Bick U, Fahrendorf G, Ludolph AC, Vassallo P, Weglage J, Ullrich K 1991 Disturbed myelination in patients with treated hyperphenylalaninaemia: evaluation with magnetic resonance imaging. Eur J Pediatr 150: 185–189
Lou H, Toft PB, Andersen J, Mikkelsen I, Olsen B, Guttler F, Wieslander S, Henriksen O 1992 An occipito-temporal syndrome in adolescents with optimally controlled hyperphenylalaninaemia. J Inhert Metab Dis 15: 687–695
Thompson AJ, Tillotson S, Smith I, Kendall B, Moore SG, Brenton D 1993 Brain magnetic resonance imaging changes in phenylketonuria. Brain 116: 811–821
Johannik K, Van Hecke P, François B, Marchal G, Smet M-H, Jaeken J, Breysem L, Wilms G, Baert AL 1994 Localized brain proton NMR spectroscopy in young adult phenylketonuria patients. Magn Reson Med 31: 53–57
Novotny EJ Jr, Avison MJ, Herschkowitz N, Petroff OAC, Prichard JW, Seashore MR, Rothman DL 1995 In vivo measurement of phenylalanine in human brain by proton nuclear magnetic resonance spectroscopy. Pediatr Res 37: 244–249
Lou HC 1985 Large doses of tryptophan and tyrosine as potential therapeutic alternative to dietary phenylalanine restriction in phenylketonuria. Lancet 2: 150–151
Schroeder T, Holstein P, Lassen NA, Engell HC 1986 Measurement of cerebral blood flow by intravenous xenon-133 technique and a mobile system. Neurol Res 8: 237–242
Cooke BE, Evans AC, Fanthome EO, Alerie E, Sendyk AM 1984 Performance figures and images from the Therascan 3128 positron emission tomograph. IEEE Trans Nucl Sci NS 31: 640–644
Sokoloff L, Reivich M, Kennedy C, DesRosiers MH, Patlak CS, Pettigrew KD, Sakurada D, Shinohara M 1977 The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28: 897–916
Brooks RA 1982 Alternative formula for glucose utilisation using labelled deoxyglucose. J Nucl Med 23: 538–539
Aquilonius SM, Eckernäs SÅ 1980 A Colour Atlas of the Human Brain. Adapted to Computed Tomography. Esselte Studium, Stockholm
Waldemar G, Hasselbalch S, Andersen AR, Delecluse F, Petersen P, Johnsen A, Paulson OB 1991 99mTc-d,l-HMPAO and SPECT of the brain in normal aging. J Cereb Blood Flow Metab 11: 508–521
Wang G-J, Volkow ND, Wolf AP, Brodie JD, Hitzemann RJ 1994 Intersubject variability of brain glucose metabolic measurements in young normal males. J Nucl Med 35: 1457–1466
Haier RJ, Siegel BV, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS 1992 Regional glucose metabolic changes after learning a complex visuospatial/motor task: a positron emission tomographic study. Brain Res 570: 134–143
Knudsen GM, Hasselbalch S, Toft P, Christensen E, Lou H, Paulson OB 1995 Blood-brain barrier transport of amino acids in healthy controls and in patients with phenylketonuria. J Inherited Metab Dis 18: 653–664
Author information
Authors and Affiliations
Additional information
Supported by the Danish Health Research Council and by the Lundbeck Foundation.
Rights and permissions
About this article
Cite this article
Hasselbalch, S., Knudsen, G., Toft, P. et al. Cerebral Glucose Metabolism Is Decreased in White Matter Changes in Patients with Phenylketonuria. Pediatr Res 40, 21–24 (1996). https://doi.org/10.1203/00006450-199607000-00004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199607000-00004
This article is cited by
-
Phenylketonuria
Nature Reviews Disease Primers (2021)
-
Biochemical, Metabolic, and Behavioral Characteristics of Immature Chronic Hyperphenylalanemic Rats
Neurochemical Research (2016)
-
Pyruvate and creatine prevent oxidative stress and behavioral alterations caused by phenylalanine administration into hippocampus of rats
Metabolic Brain Disease (2012)
-
Oxidative Stress in Phenylketonuria: What is the Evidence?
Cellular and Molecular Neurobiology (2011)
-
Experimental Evidence that Phenylalanine Provokes Oxidative Stress in Hippocampus and Cerebral Cortex of Developing Rats
Cellular and Molecular Neurobiology (2010)