Abstract
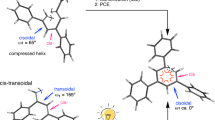
A new polyacetylene bearing benzylidene malonate group, poly[4-{(diethyl-β-malonyl)vinyl}phenylacetylene], 1, was designed as a prochiral reacting polyacetylene intermediate to provide helix induction and inversion abilities with chiral nucleophiles. Polymer 1 underwent both Michael reaction and amidation in the presence of (R)-2-amino-1-propanol (R)-3 (and its (S)-form) to afford optically active polymers with extremely complicated and optically active sites in the side chains. UV-vis, circular dichroism, and NMR studies of 1 revealed the rapid coil-to-helix and subsequent slow helix-to-helix transitions of the resulting optically active polymers. Although the inverted helical conformation was fairly stabilized by intramolecular hydrogen bonds between the complicated and optically active side chains, the inverted helical sense was readily recovered to the initial screw sense by adding a very little amount of water. Polymer 1 is applicable to new chiroptical switching induced by various chiral nucleophiles and sensory systems to detect a trace amount of water. Polymer 1 may thus be a very useful prochiral precursor polymer for preparing various functional and biomimetic helical and higher ordered polymers in future.
Similar content being viewed by others
Article PDF
References
C. F. Bernasconi and M. W. Stronach, J. Am. Chem. Soc., 113, 2222 (1991).
D. A. Evans, T. Rovis, M. C. Kozlowski, and J. S. Tedrow, J. Am. Chem. Soc., 121, 1994 (1999).
W. Zhuang, T. Hansen, and K. A. Jørgensen, Chem. Commun., 347 (2001).
G. Cardillo, L. Gentilucci, M. Gianotti, H. Kim, R. Perciaccante, and A. Tolomelli, Tetrahedron: Asymmetry, 12, 2395 (2001).
J. M. Betancort, K. Sakthivel, R. Thayumanavan, and C. F. Barbas III, Tetrahedron Lett., 42, 4441 (2001).
G. Cardillo, S. Fabbroni, L. Gentilucci, M. Gianotti, R. Perciaccante, and A. Tolomelli, Tetrahedron: Asymmetry, 13, 1407 (2002).
J. Zhou and Y. Tang, J. Am. Chem. Soc., 124, 9030 (2002).
J. J. L. M. Cornelissen, J. J. J. M. Donners, R. de Gelder, W. S. Graswinckel, G. A. Metselaar, A. E. Rowan, N. A. J. M. Sommerdijk, and R. J. M. Nolte, Science, 293, 676 (2001).
R. Nomura, J. Tabei, and T. Masuda, J. Am. Chem. Soc., 123, 8430 (2001).
H. Onouchi, K. Maeda, and E. Yashima, J. Am. Chem. Soc., 123, 7441 (2001).
B. S. Li, K. K. L. Cheuk, F. Salhi, J. W. Y. Lam, J. A. K. Cha, X. Xiao, C. Bai, and B. Z. Tang, Nano Lett., 1, 323 (2001).
R. Nonokawa and E. Yashima, J. Am. Chem. Soc., 125, 1278 (2002).
T. Aoki, T. Kaneko, N. Maruyama, A. Sumi, M. Takahashi, T. Sato, and M. Teraguchi, J. Am. Chem. Soc., 125, 6346 (2003).
K. K. L. Cheuk, J. W. Y. Lam, J. Chen, L. M. Lai, and B. Z. Tang, Macromolecules, 36, 5947 (2003).
J. Tabei, R. Nomura, and T. Masuda, Macromolecules, 36, 573 (2003).
K. K. L. Cheuk, J. W. Y. Lam, L. M. Lai, Y. Dong, and B. Z. Tang, Macromolecules, 36, 9752 (2003).
J. Tabei, R. Nomura, F. Sanda, and T. Masuda, Macromolecules, 37, 1175 (2004).
H. Nakashima, J. R. Koe, K. Torimitsu, and M. Fujiki, J. Am. Chem. Soc., 123, 4847 (2001).
H. Onouchi, T. Miyagawa, A. Furuko, K. Maeda, and E. Yashima, J. Am. Chem. Soc., 127, 2960 (2005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kwak, G., Hososhima, SI. & Fujiki, M. Polyacetylene Intermediate Bearing Reactive Benzylidene Malonate: Helix Induction, Inversion, and Recovery by Tandem Michael and Amidation Reactions with Chiral Nucleophiles and Water. Polym J 38, 976–982 (2006). https://doi.org/10.1295/polymj.PJ2006027
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1295/polymj.PJ2006027