Abstract
Background:
Recent literature has suggested that bicycling may be associated with increases in serum PSA levels, a diagnostic and prognostic marker for prostate cancer. To further investigate this relationship, we conducted a systematic review and meta-analysis of current literature in this field.
Methods:
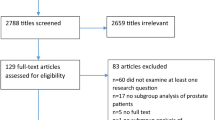
MEDLINE, CENTRAL, CINAHL and SPORTDiscus were searched using MeSH terms and keywords for English publications related to bicycle riding and PSA. Studies were included if PSA was measured relative to cycling activity in healthy men who were free of any prostatic condition. Case studies were excluded.
Results:
Eight studies met our inclusion criteria, comprising 912 participants that engaged in, or self-reported, bicycling activity. Six studies investigated the acute pre-post change in PSA following bicycling activity that ranged from a single cycling bout of 15 min to a 4-day cycling event. Following cycling activity, two studies reported total PSA increased from baseline by up to 3.3-fold, free PSA increased in one study by 0.08±0.18 ng ml−1 and did not change in four studies. One study compared PSA in elite/professional cyclists versus non-cyclists and demonstrated no significant difference in PSA measurements between groups. Data from six studies were meta-analyzed and demonstrated no significant increase in PSA associated with cycling from pre to post (mean change +0.027 ng ml−1, s.e.m.=0.08, P=0.74, 95% confidence interval (CI)=−0.17–0.23).
Conclusions:
Our findings suggest that there is no effect of cycling on PSA; however, the limited number of trials and the absence of randomized controlled trials limit the interpretation of our results. Additionally, the median sample size only consisted of 42 subjects. Therefore, our study may have low statistical power to detect a difference in PSA. Although, a higher sample size may demonstrate statistical significance, it may not be clinically significant. Studies of higher empirical quality are needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Leibovitch I, Mor Y . The vicious cycling: bicycling related urogenital disorders. Eur Urol 2005; 47: 277–l86; discussion 286–7.
Oremek GM, Seiffert UB . Physical activity releases prostate-specific antigen (PSA) from the prostate gland into blood and increases serum PSA concentrations. Clin Chem 1996; 42: 691–695.
Safford RH . The effect of bicycle riding on serum prostate specific antigen levels. Am Urol Assoc 1996; 156: 103–105.
Mejak SL, Bayliss J, Hanks SD . Long distance bicycle riding causes prostate-specific antigen to increase in men aged 50 years and over. PLoS ONE 2013; 8: e56030.
Nadler RB, Humphrey PA, Smith DS, Catalona WJ, Ratliff TL . Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol 1995; 154: 407–413.
Swain RA, Montalto N, Ross D . The effect of long-distance cycling on the prostate-specific antigen level. Arch Fam Med 1997; 6: 500–502.
Luboldt H-J, Peck KD, Oberpenning F, Schmid H-P, Semjonow A . Bicycle riding has no important impact on total and free prostate-specific antigen serum levels in older men. Urology 2003; 61: 1177–1180.
Herrmann M, Scharhag J, Sand-Hill M, Kindermann W, Herrmann W . Long-distance mountain biking does not disturb the measurement of total, free or complexed prostate-specific antigen in healthy men. Clin Chem Lab Med 2004; 42: 347–349.
Lippi G, Corgnati a, Salvagno G, Schena F, Franchini M, Guidi G . Total and free PSA serum concentrations are not influenced by extensive physical exercise and bicycle riding. Int J Sports Med 2005; 26: 79–81.
Saka T, Sofikerim M, Demirtas A, Kulaksizoglu S, Caniklioglu M, Karacagil M . Rigorous bicycling does not increase serum levels of total and free prostate-specific antigen (PSA), the free/total PSA ratio, gonadotropin levels, or uroflowmetric parameters. Urology 2009; 74: 1325–1330.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD et al. The Cochrane Collaboration's tool for assessing risk of bias in randomized trials. BMJ 2011; 343: d5928.
Arneth BM . Clinical significance of measuring prostate-specific antigen. Lab Med 2009; 40: 487–491.
Cevik I, Türkeri LN, Ozveri H, Ilker Y, Akdaş A . Short-term effect of digital rectal examination on serum prostate-specific antigen levels. A prospective study. Eur Urol 1996; 29: 403–406.
Ornstein DK, Smith DS, Humphrey Pa, Catalona WJ . The effect of prostate volume, age, total prostate specific antigen level and acute inflammation on the percentage of free serum prostate specific antigen levels in men without clinically detectable prostate cancer. J Urol 1998; 159: 1234–1237.
Ornstein DK, Rao G, Smith D . Effect of digital rectal examination and needle biopsy on serum total and percentage of free prostate specific antigen levels. J Urol 1997; 157: 195–198.
Collins GN, Martin PJ, Wynn-davies A, Brooman PJ, Reilly PHO . The effect of digital rectal examination, flexible cystoscopy and prostatic biopsy on free and total prostate. Eur Urol 1997; 157: 1744–1747.
Glenski WJ, Oesterling JE, Klee GG, Bergstralh EJ . Prostate-specific antigen: Establishment of the reference range for the clinically normal prostate gland and the effect of digital rectal examination, ejaculation, and time on serum concentrations. Prostate 1992; 21: 99–110.
McAleer JK, Gerson LW, McMahon D, Geller L . Effect of digital rectal examination (and ejaculation) on serum prostate-specific antigen after twenty-four hours. A randomized, prospective study. Urology 1993; 41: 111–112.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jiandani, D., Randhawa, A., Brown, R. et al. The effect of bicycling on PSA levels: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 18, 208–212 (2015). https://doi.org/10.1038/pcan.2015.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2015.16
This article is cited by
-
Case report. Het effect van fietsen op totaal-PSA: een update
Tijdschrift voor Urologie (2016)