Abstract
Background:
Due to concerns of fragility fracture, exercise is a perceived contraindication for prostate cancer patients with bone metastases. These patients experience significant functional impairment and muscle atrophy, which may lead to an increased likelihood of skeletal complications (i.e., pathological fracture, bone pain) and/or falls. Safe resistance exercise prescription may counteract this effect. The aim of this feasibility trial was to determine the safety and efficacy of resistance exercise by prostate cancer survivors with bone metastatic disease.
Methods:
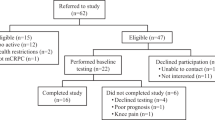
Twenty men with established bone metastases secondary to prostate cancer were randomly assigned to a 12-week resistance exercise program in which exercise prescription was based on the location of bone lesions (n=10) or usual care (n=10). Outcomes included safety and tolerance of the exercise program, physical function, physical activity level, body composition, fatigue, quality of life and psychological distress. Outcomes were compared between groups using analysis of covariance adjusted for baseline values.
Results:
Participants had significant disease load with 65% of participants presenting with two or more regions affected by bone metastases and an average Gleason score of 8.2±0.9. Five participants (exercise=2; usual care=3) did not complete the intervention, three of which were due to advancing disease (exercise=2; usual care=1). No adverse events or skeletal complications occurred during the supervised exercise sessions. The exercise program was well tolerated as evidenced by high attendance (83%) and compliance rates (93%), and the ability of the participants to exercise at an intensity within the target range for cancer survivors (rating of perceived exertion =13.8±1.5). The change in physical function (muscle strength ∼11%; submaximal aerobic exercise capacity ∼5% and ambulation ∼12%), physical activity level (∼24%) and lean mass (∼3%) differed significantly between groups following the intervention, with favorable changes in the exercise group compared with the usual care group. No significant between-group differences were observed for fatigue, quality of life or psychological distress.
Conclusions:
This initial evidence involving a small sample size suggests that appropriately designed and supervised resistance exercise may be safe and well tolerated by prostate cancer patients with bone metastatic disease and can lead to improvements in physical function, physical activity levels and lean mass. Future trials involving larger sample sizes are required to expand these preliminary findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Coleman RE . Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27: 165–176.
Lee RJ, Saylor PJ, Smith MR . Treatment and prevention of bone complications from prostate cancer. Bone 2011; 48: 88–95.
Jemal A, Siegel R, Xu J, Ward E . Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300.
Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005; 16: 579–584.
Carlin BI, Andriole GL . The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer 2000; 88 (12 Suppl): 2989–2994.
Sharifi N, Gulley JL, Dahut WL . Androgen deprivation therapy for prostate cancer. JAMA 2005; 294: 238–244.
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS . Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005; 352: 154–164.
Galvao DA, Spry NA, Taaffe DR, Newton RU, Stanley J, Shannon T et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int 2008; 102: 44–47.
Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, Keating NL et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 2010; 121: 833–840.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr., Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520.
Saad F, Olsson C, Schulman CC . Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur Urol 2004; 46: 731–739.
Eton DT, Lepore SJ . Prostate cancer and health-related quality of life: a review of the literature. Psychooncology 2002; 11: 307–326.
Galvao DA, Taaffe DR, Spry N, Newton RU . Physical activity and genitourinary cancer survivorship. Recent Results Cancer Res 2011; 186: 217–236.
Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM . Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol 2011; 29: 726–732.
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012; 62: 242–274.
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010; 42: 1409–1426.
Courneya KS, Friedenreich CM (eds). Physical Activity and Cancer. Springer: London, 2011; p 387.
Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU . Cardiovascular and metabolic complications during androgen deprivation: exercise as a potential countermeasure. Prostate Cancer Prostatic Dis 2009; 12: 233–240.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). U.S. Department of Health and Human Services, National Institute of Health, 2009; p 196.
Galvao DA, Taaffe DR, Cormie P, Spry N, Chambers SK, Peddle-McIntyre C et al. Efficacy and safety of a modular multi-modal exercise program in prostate cancer patients with bone metastases: a randomized controlled trial. BMC Cancer 2011; 11: 517.
Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU . Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010; 28: 340–347.
Broom R, Du H, Clemons M, Eton D, Dranitsaris G, Simmons C et al. Switching breast cancer patients with progressive bone metastases to third-generation bisphosphonates: measuring impact using the functional assessment of cancer therapy-bone pain. J Pain Symptom Manage 2009; 38: 244–257.
Popovic M, Nguyen J, Chen E, Di Giovanni J, Zeng L, Chow E . Comparison of the EORTC QLQ-BM22 and the FACT-BP for assessment of quality of life in cancer patients with bone metastases. Expert Rev Pharmacoecon Outcomes Res 2012; 12: 213–219.
Price DD, McGrath PA, Rafii A, Buckingham B . The validation of visual analog scales as ratio scale measures for chronic and experimental pain. Pain 1983; 17: 45–56.
Borg G . Borg's Perceived Exertion and Pain Scales. Human Kinetics: Champaign: IL, USA, 1998; pp 1–104.
Cormie P, Galvao DA, Spry N, Newton RU . Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr Cancer Ther 2013.
Myers AM, Fletcher PC, Myers AH, Sherk W . Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1998; 53: M287–M294.
Sirard JR, Forsyth A, Oakes JM, Schmitz KH . Accelerometer test-retest reliability by data processing algorithms: results from the Twin Cities Walking Study. J Phys Act Health 2011; 8: 668–674.
Freedson PS, Melanson E, Sirard J . Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports E 1998; 30: 777–781.
Godin G, Shephard RJ . A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985; 10: 141–146.
Jacobs DR Jr., Ainsworth BE, Hartman TJ, Leon AS . A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc 1993; 25: 81–91.
Stein KD, Jacobsen PB, Blanchard CM, Thors C . Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004; 27: 14–23.
Ware JE Jr., Sherbourne CD . The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483.
Zabora J, BrintzenhofeSzoc K, Jacobsen P, Curbow B, Piantadosi S, Hooker C et al. A new psychosocial screening instrument for use with cancer patients. Psychosomatics 2001; 42: 241–246.
Liu CJ, Latham NK . Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; 3: 267.
Oefelein MG, Ricchiuti V, Conrad W, Resnick MI . Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 2002; 168: 1005–1007.
Ebeling PR . Clinical practice. Osteoporosis in men. N Engl J Med 2008; 358: 1474–1482.
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012; 9: CD007146.
Hechmati G, Cure S, Gouepo A, Hoefeler H, Lorusso V, Luftner D et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ 2013; 16: 691–700.
Mavros Y, Kay S, Anderberg KA, Baker MK, Wang Y, Zhao R et al. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: Interim outcomes from the GREAT2DO Trial. Diabet Care, 8 March 2013 (Epub ahead of print).
Acknowledgements
This study was funded by the Cancer Council of Western Australia through the Early Career Investigator research grants program. PC is supported by the Cancer Council Western Australia Postdoctoral Research Fellowship. DAG is funded by a Movember New Directions Development Award obtained through the Prostate Cancer Foundation of Australia’s Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Cormie, P., Newton, R., Spry, N. et al. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis 16, 328–335 (2013). https://doi.org/10.1038/pcan.2013.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2013.22
Keywords
This article is cited by
-
Comprehensive Management of Spine Metastasis in Cancer Patients: From Identification to Rehabilitation
Current Physical Medicine and Rehabilitation Reports (2024)
-
Physical activity and pain in people with cancer: a systematic review and meta-analysis
Supportive Care in Cancer (2024)
-
Therapeutic validity and effectiveness of exercise interventions after lower limb-salvage surgery for sarcoma: a systematic review
BMC Musculoskeletal Disorders (2023)
-
Feasibility of home-based exercise training in men with metastatic castration-resistant prostate cancer
Prostate Cancer and Prostatic Diseases (2023)
-
Feasibility of home-based exercise training during adjuvant treatment for metastatic castrate-resistant prostate cancer patients treated with an androgen receptor pathway inhibitor (EXACT)
Supportive Care in Cancer (2023)