Abstract
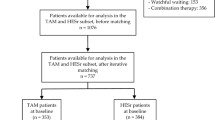
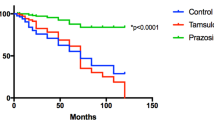
CombAT (Combination of Avodart and Tamsulosin) was a randomised, double-blind study in men (n=4844) aged ⩾50 years with a clinical diagnosis of BPH. Patients were randomised to daily tamsulosin 0.4 mg, dutasteride 0.5 mg or both for 4 years. The primary endpoint was time to acute urinary retention (AUR) or BPH-related surgery. Secondary endpoints included BPH clinical progression, symptoms and maximum urinary flow rate. A post hoc analysis of data from the European subgroup was conducted. A total of 2925 men were randomised to treatment in Europe as part of CombAT (tamsulosin, n=972; dutasteride, n=970; combination, n=983). Combination therapy significantly reduced the relative risk of AUR or BPH-related surgery compared with either monotherapy at 4 years, and also significantly reduced the risk of BPH clinical progression. Combination therapy also provided significantly greater symptom improvement than either monotherapy at 4 years. Safety and tolerability of dutasteride plus tamsulosin was consistent with previous experience of this combination and with the monotherapies. These data provide further evidence to support the use of long-term combination therapy (dutasteride plus tamsulosin) in men with moderate-to-severe lower urinary tract symptoms because of BPH and prostatic enlargement. The results in the European subgroup are generally consistent with those in the overall study population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Emberton M, Fitzpatrick JM, Garcia-Losa M, Qizilbash N, Djavan B . Progression of benign prostatic hyperplasia: systematic review of the placebo arms of clinical trials. BJU Int 2008; 102: 981–986.
Roehrborn CG, Boyle P, Bergner D, Gray T, Gittelman M, Shown T et al. Serum prostate-specific antigen and prostate volume predict long-term changes in symptoms and flow rate: results of a four-year, randomized trial comparing finasteride versus placebo. Urology 1999; 54: 662–669.
Roehrborn CG, McConnell JD, Lieber M, Kaplan S, Geller J, Malek GH et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. Urology 1999; 53: 473–480.
Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ . EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol 2004; 46: 547–554.
Roehrborn CG, McConnell JD, Barry MJ, Benaim E, Bruskewitz RC, Blute ML et al. AUA guideline on the management of benign prostatic hyperplasia (BPH) 2003. Updated 2006. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph. [accessed 26 October 2010].
McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Dixon CM, Kusek JW et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349: 2387–2398.
McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med 1998; 338: 557–563.
Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G . The long-term outcome of medical therapy for BPH. Eur Urol 2007; 51: 1522–1533.
Siami P, Roehrborn CG, Barkin J, Damião R, Wyczolkowski M, Duggan A et al. Combination therapy with dutasteride and tamsulosin in men with moderate-to-severe benign prostatic hyperplasia: the CombAT (Combination of Avodart and Tamsulosin) trial rationale and study design. Contemp Clin Trial 2007; 28: 770–779.
Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Morrill B et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 2008; 179: 616–621.
Barkin J, Roehrborn CG, Siami P, Haillot O, Morrill B, Black L et al. Effect of dutasteride, tamsulosin and the combination on patient-reported quality of life and treatment satisfaction in men with moderate-to-severe benign prostatic hyperplasia: 2-year data from the CombAT trial. BJU Int 2009; 103: 919–926.
Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57: 123–131.
Parsons JK . Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol 2007; 178: 395–401.
Parsons JK . Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol 2011; 21: 1–4.
Fourcade RO, Théret N, Taïeb C . Profile and management of patients treated for the first time for lower urinary tract symptoms/benign prostatic hyperplasia in four European countries. BJU Int 2008; 101: 1111–1118.
Hutchison A, Farmer R, Verhamme K, Berges R, Vela Navarrete R . The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur Urol 2007; 51: 207–216.
Acknowledgements
This study was funded by GlaxoSmithKline. Medical writing assistance in the preparation of this paper was provided by Tony Reardon of Spirit Medical Communications and funded by GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Drs Haillot, Fraga, Maciukiewicz, Pushkar, Tammela, Höfner and Chantada have been investigators in studies sponsored by GlaxoSmithKline. Dr Tammela has acted as an advisor to GlaxoSmithKline. Paul Gagnier and Betsy Morrill are employees of GlaxoSmithKline.
Rights and permissions
About this article
Cite this article
Haillot, O., Fraga, A., Maciukiewicz, P. et al. The effects of combination therapy with dutasteride plus tamsulosin on clinical outcomes in men with symptomatic BPH: 4-year post hoc analysis of European men in the CombAT study. Prostate Cancer Prostatic Dis 14, 302–306 (2011). https://doi.org/10.1038/pcan.2011.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2011.13