Abstract
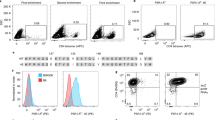
We set out to develop a PSA peptide-loaded tetramer for enumeration of PSA-specific CD8+ T cells in the Balb/c mouse model. A candidate major histocompatibility complex (MHC) class I PSA peptide (HPQKVTKFML188−197) was selected on the basis of its ability to restimulate PSA-specific CD8+ T cells to secrete interferon-γ in our assays. Next, H-2Ld-restricted peptide-loaded and fluorescently labeled tetramers were produced in conjunction with the NIH Tetramer Core Facility, Atlanta, GA, USA. This tetramer was then tested for staining specificity and optimized for detection of PSA-specific CD8+ T cells induced by our PSA-encoding adenovirus tumor vaccine. The MHC class I PSA peptide demonstrated successful restimulation of CD8+ T cells isolated from mice previously vaccinated with a PSA-encoding adenovirus tumor vaccine, with no restimulation observed in control-vaccinated mice. The peptide-loaded H-2Ld tetramer exhibited the desired binding specificity and allowed for detection and frequency determination of PSA-specific CD8+ T cells by flow cytometry. We have successfully designed and validated a PSA peptide tetramer for use in the Balb/c mouse model that can be used to test PSA-based prostate cancer vaccines. Until now, PSA-specific CD8+ T cells in the mouse have only been detectable via cytotoxic T-lymphocyte assays or intracellular cytokine staining, which primarily assess antigen-specific functional activity and not their absolute number. This research tool provides laboratories the ability to directly quantitate CD8+ T cells elicited by PSA-specific immunotherapies and cancer vaccines that are tested in mouse models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Melief CJ . Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res 1992; 58: 143–175.
Engleman EG, Brody J, Soares L . Using signaling pathways to overcome immune tolerance to tumors. Sci STKE 2004; 2004: pe28.
Furumoto K, Soares L, Engleman EG, Merad M . Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest 2004; 113: 774–783.
Morris E, Hart D, Gao L, Tsallios A, Xue SA, Stauss H . Generation of tumor-specific T-cell therapies. Blood Rev 2006; 20: 61–69.
Blohm U, Potthoff D, van der Kogel AJ, Pircher H . Solid tumors ‘melt’ from the inside after successful CD8 T cell attack. Eur J Immunol 2006; 36: 468–477.
Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S . In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med 2007; 204: 345–356.
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME . Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8: 299–308.
Saha A, Bhattacharya-Chatterjee M, Foon KA, Celis E, Chatterjee SK . Stimulatory effects of CpG oligodeoxynucleotide on dendritic cell-based immunotherapy of colon cancer in CEA/HLA-A2 transgenic mice. Int J Cancer 2009; 124: 877–888.
Davila E, Celis E . Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J Immunol 2000; 165: 539–547.
Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst 1998; 90: 1894–1900.
Siemens DR, Elzey BD, Lubaroff DM, Bohlken C, Jensen RJ, Swanson AK et al. Cutting edge: restoration of the ability to generate CTL in mice immune to adenovirus by delivery of virus in a collagen-based matrix. J Immunol 2001; 166: 731–735.
Elzey BD, Siemens DR, Ratliff TL, Lubaroff DM . Immunization with type 5 adenovirus recombinant for a tumor antigen in combination with recombinant canarypox virus (ALVAC) cytokine gene delivery induces destruction of established prostate tumors. Int J Cancer 2001; 94: 842–849.
Miller G, Lahrs S, Pillarisetty VG, Shah AB, DeMatteo RP . Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell-mediated tumor protection. Cancer Res 2002; 62: 5260–5266.
Lubaroff DM, Karan D, Andrews MP, Acosta A, Abouassaly C, Sharma M et al. Decreased cytotoxic T cell activity generated by co-administration of PSA vaccine and CpG ODN is associated with increased tumor protection in a mouse model of prostate cancer. Vaccine 2006; 24: 6155–6162.
Karan D, Krieg AM, Lubaroff DM . Paradoxical enhancement of CD8 T cell-dependent anti-tumor protection despite reduced CD8 T cell responses with addition of a TLR9 agonist to a tumor vaccine. Int J Cancer 2007; 121: 1520–1528.
Sorensen MR, Holst PJ, Pircher H, Christensen JP, Thomsen AR . Vaccination with an adenoviral vector encoding the tumor antigen directly linked to invariant chain induces potent CD4(+) T-cell-independent CD8(+) T-cell-mediated tumor control. Eur J Immunol 2009; 39: 2725–2736.
Lubaroff DM, Karan D . CpG oligonucleotide as an adjuvant for the treatment of prostate cancer. Adv Drug Deliv Rev 2009; 61: 268–274.
Drake CG . Immunotherapy for prostate cancer: an emerging treatment modality. Urol Clin North Am 2010; 37: 121–129, Table of Contents.
Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 1996; 274: 94–96.
Turner MJ, Abdul-Alim CS, Willis RA, Fisher TL, Lord EM, Frelinger JG . T-cell antigen discovery (T-CAD) assay: a novel technique for identifying T cell epitopes. J Immunol Methods 2001; 256: 107–119.
Marshall DJ, San Mateo LR, Rudnick KA, McCarthy SG, Harris MC, McCauley C et al. Induction of Th1-type immunity and tumor protection with a prostate-specific antigen DNA vaccine. Cancer Immunol Immunother 2005; 54: 1082–1094.
Rammensee HG . Chemistry of peptides associated with MHC class I and class II molecules. Curr Opin Immunol 1995; 7: 85–96.
Demant P, Ivanyi D, Oudshoorn-Snoek M, Calafat J, Roos MH . Molecular heterogeneity of H-2 antigens. Immunol Rev 1981; 60: 5–22.
Altman JD, Davis MM . MHC-peptide tetramers to visualize antigen-specific T cells. Curr Protoc Immunol 2003 Chapter 17: Unit 17.13.
Kim JJ, Yang JS, Dang K, Manson KH, Weiner DB . Engineering enhancement of immune responses to DNA-based vaccines in a prostate cancer model in rhesus macaques through the use of cytokine gene adjuvants. Clin Cancer Res 2001; 7: 882s–889s.
Acknowledgements
We gratefully acknowledge support from the American Cancer Society (RSG-09-015-01-CDD), the National Cancer Institute at the National Institutes of Health (1R21CA13345-01/UI Mayo Clinic Lymphoma SPORE), and the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation. C Lemke acknowledges support from the PhRMA foundation for a post-doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lemke, C., Graham, J., Lubaroff, D. et al. Development of an MHC class I Ld-restricted PSA peptide-loaded tetramer for detection of PSA-specific CD8+ T cells in the mouse. Prostate Cancer Prostatic Dis 14, 118–121 (2011). https://doi.org/10.1038/pcan.2010.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2010.57
Keywords
This article is cited by
-
Identification of swine influenza virus epitopes and analysis of multiple specificities expressed by cytotoxic T cell subsets
Virology Journal (2014)
-
Applying biodegradable particles to enhance cancer vaccine efficacy
Immunologic Research (2014)
-
Prostate Cancer and Immunoproteome: Awakening and Reprogramming the Guardian Angels
Archivum Immunologiae et Therapiae Experimentalis (2012)