Abstract
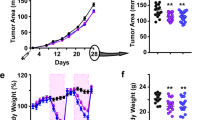
Caloric restriction (CR) has been shown to have anti-cancer properties. However, CR may be difficult to apply in humans secondary to compliance and potentially deleterious effects. An alternative is intermittent CR, or in the extreme case intermittent fasting (IF). In a previous small pilot study, we found 2 days per week of IF with ad libitum feeding on the other days resulted in trends toward prolonged survival of mice bearing prostate cancer xenografts. We sought to confirm these findings in a larger study. A total of 100 (7- to 8-week-old) male severe combined immunodeficiency mice were injected subcutaneously with 1 × 105 LAPC-4 prostate cancer cells. Mice were randomized to either ad libitum Western Diet (44% carbohydrates, 40% fat and 16% protein) or ad libitum Western Diet with twice-weekly 24 h fasts (IF). Tumor volumes and mouse bodyweights were measured twice weekly. Mice were killed when tumor volumes reached 1000 mm3. Serum and tumor were collected for analysis of the insulin/insulin-like growth factor 1 (IGF-1) hormonal axis. Overall, there was no difference in mouse survival (P=0.37) or tumor volumes (P⩾0.10) between groups. Mouse body weights were similar between arms (P=0.84). IF mice had significantly higher serum IGF-1 levels and IGF-1/IGFBP-3 ratios at killing (P<0.001). However, no difference was observed in serum insulin, IGFBP-3 or tumor phospho-Akt levels (P⩾0.39). IF did not improve mouse survival nor did it delay prostate tumor growth. This may be secondary to metabolic adaptations to the 24 h fasting periods. Future studies are required to optimize CR for application in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009; 325: 201–204.
Heilbronn LK, Ravussin E . Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 2003; 78: 361–369.
Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS . Dietary restriction and life-span. Science 2002; 296: 2141–2142;author reply 2141-2.
Lin SJ, Defossez PA, Guarente L . Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000; 289: 2126–2128.
Li Y, Chen K, Yao Q, Li J, Wang Y, Liu H et al. The effect of calorie restriction on growth and development in silkworm, Bombyx mori. Arch Insect Biochem Physiol 2009; 71: 159–172.
Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP . Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol 1988; 43: B13–B21.
Beauchene RE, Bales CW, Bragg CS, Hawkins ST, Mason RL . Effect of age of initiation of feed restriction on growth, body composition, and longevity of rats. J Gerontol 1986; 41: 13–19.
Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H . Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev 1994; 76: 215–224.
Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS . Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med 1998; 25: 1089–1097.
Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H . Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev 1994; 74: 121–133.
Fontana L, Klein S . Aging, adiposity, and calorie restriction. JAMA 2007; 297: 986–994.
Fontana L, Meyer TE, Klein S, Holloszy JO . Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 2004; 101: 6659–6663.
Walford RL, Mock D, MacCallum T, Laseter JL . Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: health, aging, and toxicological perspectives. Toxicol Sci 1999; 52: 61–65.
Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, Tataranni PA et al. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr 2000; 72: 946–953.
Michels KB, Ekbom A . Caloric restriction and incidence of breast cancer. JAMA 2004; 291: 1226–1230.
Elias SG, Peeters PH, Grobbee DE, van Noord PA . Transient caloric restriction and cancer risk (the Netherlands). Cancer Causes Control 2007; 18: 1–5.
Knauper B, Cheema S, Rabiau M, Borten O . Self-set dieting rules: adherence and prediction of weight loss success. Appetite 2005; 44: 283–288.
Burke LE, Dunbar-Jacob JM, Hill MN . Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med 1997; 19: 239–263.
Miller SL, Wolfe RR . The danger of weight loss in the elderly. J Nutr Health Aging 2008; 12: 487–491.
Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol 2007; 102: 634–640.
Buschemeyer III WC, Klink JC, Mavropoulos JC, Poulton SH, Demark-Wahnefried W, Hursting SD et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate 2010; 70: 1037–1043.
Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res 2004; 64: 5232–5236.
Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grambsch PL, Grande JP et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr Cancer 2009; 61: 265–275.
Kealy RD, Lawler DF, Ballam JM, Mantz SL, Biery DN, Greeley EH et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc 2002; 220: 1315–1320.
Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002; 418: 344–348.
Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L . Dietary restriction in long-lived dwarf flies. Science 2002; 296: 319.
Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res 1997; 57: 4667–4672.
Hursting SD, Perkins SN, Brown CC, Haines DC, Phang JM . Calorie restriction induces a p53-independent delay of spontaneous carcinogenesis in p53-deficient and wild-type mice. Cancer Res 1997; 57: 2843–2846.
Hursting SD, Switzer BR, French JE, Kari FW . The growth hormone: insulin-like growth factor 1 axis is a mediator of diet restriction-induced inhibition of mononuclear cell leukemia in Fischer rats. Cancer Res 1993; 53: 2750–2757.
Mai V, Colbert LH, Berrigan D, Perkins SN, Pfeiffer R, Lavigne JA et al. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res 2003; 63: 1752–1755.
Hursting SD, Kari FW . The anti-carcinogenic effects of dietary restriction: mechanisms and future directions. Mutat Res 1999; 443: 235–249.
Pollak M . Macronutrient intake and cancer: how does dietary restriction influence tumor growth and why should we care? Cancer Prev Res 2009; 2: 698–701.
Kalaany NY, Sabatini DM . Tumours with PI3 K activation are resistant to dietary restriction. Nature 2009; 458: 725–731.
Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 1998; 279: 563–566.
Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med 2008; 149: 461–471, W83–8.
Platz EA, Pollak MN, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E . Plasma insulin-like growth factor-1 and binding protein-3 and subsequent risk of prostate cancer in the PSA era. Cancer Causes Control 2005; 16: 255–262.
Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst 2002; 94: 1099–1106.
Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grande JP, Lokshin A et al. Cross-sectional analysis of intermittent versus chronic caloric restriction in the TRAMP mouse. Prostate 2009; 15: 69:317–69:326.
Giovannucci E . Modifiable risk factors for colon cancer. Gastroenterol Clin North Am 2002; 31: 925–943.
Katz LE, Satin-Smith MS, Collett-Solberg P, Baker L, Stanley CA, Cohen P . Dual regulation of insulin-like growth factor binding protein-1 levels by insulin and cortisol during fasting. J Clin Endocrinol Metab 1998; 83: 4426–4430.
Russell-Jones DL, Bates AT, Umpleby AM, Hennessy TR, Bowes SB, Hopkins KD et al. A comparison of the effects of IGF-I and insulin on glucose metabolism, fat metabolism and the cardiovascular system in normal human volunteers. Eur J Clin Invest 1995; 25: 403–411.
Boulware SD, Tamborlane WV, Rennert NJ, Gesundheit N, Sherwin RS . Comparison of the metabolic effects of recombinant human insulin-like growth factor-I and insulin. Dose-response relationships in healthy young and middle-aged adults. J Clin Invest 1994; 93: 1131–1139.
Rajpathak SN, Gunter MJ, Wylie-Rosett J, Ho GY, Kaplan RC, Muzumdar R et al. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev 2009; 25: 3–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Thomas, J., Antonelli, J., Lloyd, J. et al. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis 13, 350–355 (2010). https://doi.org/10.1038/pcan.2010.24
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2010.24
Keywords
This article is cited by
-
PGC1 alpha coactivates ERG fusion to drive antioxidant target genes under metabolic stress
Communications Biology (2022)
-
Obesity, cancer risk, and time-restricted eating
Cancer and Metastasis Reviews (2022)
-
Avenues of research in dietary interventions to target tumor metabolism in osteosarcoma
Journal of Translational Medicine (2021)
-
Fasting and fasting-mimicking diets for chemotherapy augmentation
GeroScience (2021)
-
Physiological, mitochondrial, and oxidative stress differences in the presence or absence of lactation in rats
Reproductive Biology and Endocrinology (2018)