Abstract
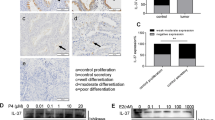
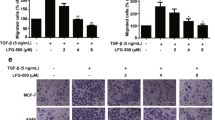
IL-37, a newly found anti-inflammatory cytokine of the IL-1 family, has both extracellular and intracellular functions. Accumulating evidences indicate that it is also involved in tumor progression. However, the mechanism and its intracellular target are unclear. In this study, clinical data from 84 patients showed that loss or reduced expression of IL-37 in lung adenocarcinoma tissues was significantly associated with tumor metastasis. We further provided evidence that IL-37 inhibited effectively tumor metastasis in vitro and in vivo. Moreover, we uncovered a novel mechanism by which IL-37 suppressed tumor cell migration via its intracellular mature form (amino acids 46–218). Intracellular mature form of IL-37, but not its extracellular form, markedly inhibited migration of multiple kinds of tumor cells through inhibiting Rac1 activation. Mechanistically, intracellular mature IL-37 directly bound to the CAAX motif in the C-terminal hypervariable region of Rac1, and then inhibited Rac1 membrane translocation and subsequent downstream signaling. Our research identifies intracellular mature IL-37 as a novel endogenous inhibitor of Rac1. Given the crucial roles of Rac1 in tumor angiogenesis and metastasis, intracellular mature IL-37 might serve as a potential strategy for the control of Rac1 activity and tumor progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kumar S, McDonnell PC, Lehr R, Tierney L, Tzimas MN, Griswold DE et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem 2000; 275: 10308–10314.
Busfield SJ, Comrack CA, Yu G, Chickering TW, Smutko JS, Zhou H et al. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics 2000; 66: 213–216.
Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Network 2011; 22: 127–147.
Quirk S, Agrawal DK . Immunobiology of IL-37: mechanism of action and clinical perspectives. Exp Rev Clin Immunol 2014; 10: 1703–1709.
Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA . IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010; 11: 1014–1022.
Gao W, Kumar S, Lotze MT, Hanning C, Robbins PD, Gambotto A . Innate immunity mediated by the cytokine IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive and profound antitumor immunity. J Immunol 2003; 170: 107–113.
Luo Y, Cai X, Liu S, Wang S, Nold-Petry CA, Nold MF et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA 2014; 111: 15178–15183.
McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA 2011; 108: 16711–16716.
Coll-Miro M, Francos-Quijorna I, Santos-Nogueira E, Torres-Espin A, Bufler P, Dinarello CA et al. Beneficial effects of IL-37 after spinal cord injury in mice. Proc Natl Acad Sci USA 2016; 113: 1411–1416.
Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun 2014; 5: 4711.
Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci USA 2015; 112: 2497–2502.
Ye L, Jiang B, Deng J, Du J, Xiong W, Guan Y et al. IL-37 alleviates rheumatoid arthritis by suppressing IL-17 and IL-17-triggering cytokine production and limiting Th17 cell proliferation. J Immunol 2015; 194: 5110–5119.
Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog 2014; 10: e1004462.
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: its correlation with disease activity. J Transl Med 2014; 12: 69.
Lunding L, Webering S, Vock C, Schroder A, Raedler D, Schaub B et al. IL-37 requires IL-18Ralpha and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy 2015; 70: 366–373.
Bulau AM, Fink M, Maucksch C, Kappler R, Mayr D, Wagner K et al. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. Sci World J 2011; 11: 2480–2490.
Wu B, Meng K, Ji Q, Cheng M, Yu K, Zhao X et al. Interleukin-37 ameliorates myocardial ischemia/reperfusion injury in mice. Clin Exp immunol 2014; 176: 438–451.
Teng X, Hu Z, Wei X, Wang Z, Guan T, Liu N et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol 2014; 192: 1815–1823.
Chai M, Ji Q, Zhang H, Zhou Y, Yang Q, Zhou Y et al. The protective effect of interleukin-37 on vascular calcification and atherosclerosis in apolipoprotein E-deficient mice with diabetes. J Interferon Cytokine Res 2015; 35: 530–539.
Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ, Li JJ et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep 2014; 4: 5177.
Ge G, Wang A, Yang J, Chen Y, Yang J, Li Y et al. Interleukin-37 suppresses tumor growth through inhibition of angiogenesis in non-small cell lung cancer. J Exp Clin Cancer Res 2016; 35: 13.
Liu R, Tang C, Shen A, Luo H, Wei X, Zheng D et al. IL-37 suppresses hepatocellular carcinoma growth by converting pSmad3 signaling from JNK/pSmad3L/c-Myc oncogenic signaling to pSmad3C/P21 tumor-suppressive signaling. Oncotarget 2016; 7: 85079–85096.
Wang S, An W, Yao Y, Chen R, Zheng X, Yang W et al. Interleukin 37 expression inhibits STAT3 to suppress the proliferation and invasion of human cervical cancer cells. J Cancer 2015; 6: 962–969.
Kumar S, Hanning CR, Brigham-Burke MR, Rieman DJ, Lehr R, Khandekar S et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 2002; 18: 61–71.
Bulau AM, Nold MF, Li S, Nold-Petry CA, Fink M, Mansell A et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA 2014; 111: 2650–2655.
Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol 2016; 46: 1067–1081.
Abulkhir A, Samarani S, Amre D, Duval M, Haddad E, Sinnett D et al. A protective role of IL-37 in cancer: a new hope for cancer patients. J Leukocyte Biol 2016; 101: 395–406.
Nold-Petry CA, Lo CY, Rudloff I, Elgass KD, Li S, Gantier MP et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol 2015; 16: 354–365.
Bosco EE, Mulloy JC, Zheng Y . Rac1 GTPase: a ‘Rac’ of all trades. Cell Mol Life Sci 2009; 66: 370–374.
Rathinam R, Berrier A, Alahari SK . Role of Rho GTPases and their regulators in cancer progression. Front Biosci 2011; 16: 2561–2571.
Liu S, Yu M, He Y, Xiao L, Wang F, Song C et al. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatol 2008; 47: 1964–1973.
Li Z, Guo C, Liu X, Zhou C, Zhu F, Wang X et al. TIPE2 suppresses angiogenesis and non-small cell lung cancer (NSCLC) invasiveness via inhibiting Rac1 activation and VEGF expression. Oncotarget 2016; 7: 62224–62239.
Chen QY, Xu LQ, Jiao DM, Yao QH, Wang YY, Hu HZ et al. Silencing of Rac1 modifies lung cancer cell migration, invasion and actin cytoskeleton rearrangements and enhances chemosensitivity to antitumor drugs. Int J Mol Med 2011; 28: 769–776.
Lin Y, Zheng Y . Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin Drug Discov 2015; 10: 991–1010.
Zou T, Mao X, Yin J, Li X, Chen J, Zhu T et al. Emerging roles of RAC1 in treating lung cancer patients. Clin Genet 2016; 91: 520–528.
Kreis P, Barnier JV . PAK signalling in neuronal physiology. Cell Gignal 2009; 21: 384–393.
Wang CQ, Sun HT, Gao XM, Ren N, Sheng YY, Wang Z et al. Interleukin-6 enhances cancer stemness and promotes metastasis of hepatocellular carcinoma via up-regulating osteopontin expression. Am J Cancer Res 2016; 6: 1873–1889.
Wang Z, Fayngerts S, Wang P, Sun H, Johnson DS, Ruan Q et al. TIPE2 protein serves as a negative regulator of phagocytosis and oxidative burst during infection. Proc Natl Acad Sci USA 2012; 109: 15413–15418.
Smithers CC, Overduin M . Structural mechanisms and drug discovery prospects of rho GTPases. Cells 2016; 5: 2.
Yan X, Zhao J, Zhang R . Interleukin-37 mediates the antitumor activity in colon cancer through beta-catenin suppression. Oncotarget 2017; 8: 49064–49075.
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo C et al. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer 2013; 12: 149.
Cherfils J, Zeghouf M . Chronicles of the GTPase switch. Nat Chem Biol 2011; 7: 493–495.
Karnoub AE, Symons M, Campbell SL, Der CJ . Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res Treat 2004; 84: 61–71.
Cherfils J, Zeghouf M . Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93: 269–309.
Wittinghofer A, Vetter IR . Structure-function relationships of the G domain, a canonical switch motif. Annu Rev Biochem 2011; 80: 943–971.
Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR . Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol 2001; 152: 111–126.
Lanning CC, Daddona JL, Ruiz-Velasco R, Shafer SH, Williams CL . The Rac1 C-terminal polybasic region regulates the nuclear localization and protein degradation of Rac1. J Biol Chem 2004; 279: 44197–44210.
Modha R, Campbell LJ, Nietlispach D, Buhecha HR, Owen D, Mott HR . The Rac1 polybasic region is required for interaction with its effector PRK1. J Biol Chem 2008; 283: 1492–1500.
Schaefer A, Reinhard NR, Hordijk PL . Toward understanding RhoGTPase specificity: structure, function and local activation. Small GTPases 2014; 5: 6.
Gao J, Liao J, Yang GY . CAAX-box protein, prenylation process and carcinogenesis. Am J Transl Res 2009; 1: 312–325.
Chen YH, Zhou BY, Wu XJ, Xu JF, Zhang JA, Chen YH et al. CCL22 and IL-37 inhibit the proliferation and epithelial-mesenchymal transition process of NSCLC A549 cells. Oncol Rep 2016; 36: 2017–2024.
Fang D, Chen H, Zhu JY, Wang W, Teng Y, Ding HF et al. Epithelial–mesenchymal transition of ovarian cancer cells is sustained by Rac1 through simultaneous activation of MEK1/2 and Src signaling pathways. Oncogene 2017; 36: 1546–1558.
Acknowledgements
This research is supported by the national key research and development plan of China (No. 2016YFC1303405), the National Natural Science Foundation of China (31470856 and 91439124), Shandong Provincial Natural Science Foundation, China (2014GSF118076, ZR2011HZ003), the Fundamental Research Funds of Shandong University (2014QY004) and Major Project of Science and Technology of Shandong Province (2015ZDJS04001). WC is supported by the Intramural Research Program of NIH, NIDCR, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Y., Zhao, M., Guo, C. et al. Intracellular mature IL-37 suppresses tumor metastasis via inhibiting Rac1 activation. Oncogene 37, 1095–1106 (2018). https://doi.org/10.1038/onc.2017.405
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.405
This article is cited by
-
Interleukin-37 is involved in the immunopathogenesis of infectious mononucleosis
Italian Journal of Pediatrics (2023)
-
Intracellular CYTL1, a novel tumor suppressor, stabilizes NDUFV1 to inhibit metabolic reprogramming in breast cancer
Signal Transduction and Targeted Therapy (2022)
-
IL-37bΔ1-45 suppresses the migration and invasion of endometrial cancer cells by targeting the Rac1/NF-κB/MMP2 signal pathway
Laboratory Investigation (2021)
-
LPCAT1 reprogramming cholesterol metabolism promotes the progression of esophageal squamous cell carcinoma
Cell Death & Disease (2021)