Abstract
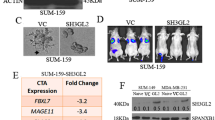
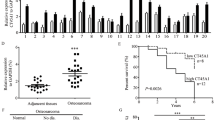
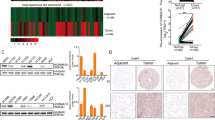
The biological function of MAGEC2, a cancer/testis antigen highly expressed in various cancers, remains largely unknown. Here we demonstrate that expression of MAGEC2 induces rounded morphology and amoeboid-like movement of tumor cells in vitro and promotes tumor metastasis in vivo. The pro-metastasis effect of MAGEC2 was mediated by signal transducer and activator of transcription 3 (STAT3) activation. Mechanistically, MAGEC2 interacts with STAT3 and inhibits the polyubiquitination and proteasomal degradation of STAT3 in the nucleus of tumor cells, resulting in accumulation of phosphorylated STAT3 and enhanced transcriptional activity. Notably, expression levels of MAGEC2 and phosphorylated STAT3 are positively correlated and both are associated with incidence of metastasis in human hepatocellular carcinoma. This study not only reveals a previously unappreciated role of MAGEC2 in promoting tumor metastasis, but also identifies a new molecular mechanism by which MAGEC2 sustains hyperactivation of STAT3 in the nucleus of tumor cells. Thus, MAGEC2 may represent a new antitumor metastasis target for treatment of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chaffer CL, Weinberg RA . A perspective on cancer cell metastasis. Science 2011; 331: 1559–1564.
Valastyan S, Weinberg RA . Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147: 275–292.
Sahai E, Marshall CJ . Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 2003; 5: 711–719.
Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E . ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol 2006; 16: 1515–1523.
Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008; 135: 510–523.
Liu YJ, Le Berre M, Lautenschlaeger F, Maiuri P, Callan-Jones A, Heuze M et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 2015; 160: 659–672.
Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell 2011; 20: 229–245.
Pinner S, Sahai E . Imaging amoeboid cancer cell motility in vivo. J Microsc 2008; 231: 441–445.
Gadea G, de Toledo M, Anguille C, Roux P . Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol 2007; 178: 23–30.
Devarajan E, Huang S . STAT3 as a central regulator of tumor metastases. Curr Mol Med 2009; 9: 626–633.
Jing N, Tweardy DJ . Targeting Stat3 in cancer therapy. Anticancer Drugs 2005; 16: 601–607.
Wegenka UM, Buschmann J, Lutticken C, Heinrich PC, Horn F . Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol 1993; 13: 276–288.
Yu H, Jove R . The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 2004; 4: 97–105.
Bromberg J . Stat proteins and oncogenesis. J Clin Invest 2002; 109: 1139–1142.
Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci 2009; 1171: 59–76.
Johnston PA, Grandis JR . STAT3 signaling: anticancer strategies and challenges. Mol Interv 2011; 11: 18–26.
Weon JL, Potts PR . The MAGE protein family and cancer. Curr Opin Cell Biol 2015; 37: 1–8.
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ . Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 2005; 5: 615–625.
Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S . An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res 2001; 61: 5544–5551.
Ayyoub M, Scarlata CM, Hamai A, Pignon P, Valmori D . Expression of MAGE-A3/6 in primary breast cancer is associated with hormone receptor negative status, high histologic grade, and poor survival. J Immunother 2014; 37: 73–76.
Yang F, Zhou X, Miao X, Zhang T, Hang X, Tie R et al. MAGEC2, an epithelial-mesenchymal transition inducer, is associated with breast cancer metastasis. Breast Cancer Res Treat 2014; 145: 23–32.
Yin B, Zeng Y, Liu G, Wang X, Wang P, Song Y . MAGE-A3 is highly expressed in a cancer stem cell-like side population of bladder cancer cells. Int J Clin Exp Pathol 2014; 7: 2934–2941.
Curioni-Fontecedro A, Nuber N, Mihic-Probst D, Seifert B, Soldini D, Dummer R et al. Expression of MAGE-C1/CT7 and MAGE-C2/CT10 predicts lymph node metastasis in melanoma patients. PLoS One 2011; 6: e21418.
von Boehmer L, Keller L, Mortezavi A, Provenzano M, Sais G, Hermanns T et al. MAGE-C2/CT10 protein expression is an independent predictor of recurrence in prostate cancer. PLoS One 2011; 6: e21366.
Pankova K, Rosel D, Novotny M, Brabek J . The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell Mol Life Sci 2010; 67: 63–71.
Hao J, Song X, Wang J, Guo C, Li Y, Li B et al. Cancer-testis antigen MAGE-C2 binds Rbx1 and inhibits ubiquitin ligase-mediated turnover of cyclin E. Oncotarget 2015; 6: 42028–42039.
Doyle JM, Gao J, Wang J, Yang M, Potts PR . MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell 2010; 39: 963–974.
Tanaka T, Yamamoto Y, Muromoto R, Ikeda O, Sekine Y, Grusby MJ et al. PDLIM2 inhibits T helper 17 cell development and granulomatous inflammation through degradation of STAT3. Sci Signal 2011; 4: ra85.
Yamamoto T, Sekine Y, Kashima K, Kubota A, Sato N, Aoki N et al. The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem Biophys Res Commun 2002; 297: 811–817.
Wang Y, Ning H, Ren F, Zhang Y, Rong Y, Su F et al. GdX/UBL4A specifically stabilizes the TC45/STAT3 association and promotes dephosphorylation of STAT3 to repress tumorigenesis. Mol Cell 2014; 53: 752–765.
Riener MO, Wild PJ, Soll C, Knuth A, Jin B, Jungbluth A et al. Frequent expression of the novel cancer testis antigen MAGE-C2/CT-10 in hepatocellular carcinoma. Int J Cancer 2009; 124: 352–357.
Li B, Qian XP, Pang XW, Zou WZ, Wang YP, Wu HY et al. HCA587 antigen expression in normal tissues and cancers: correlation with tumor differentiation in hepatocellular carcinoma. Lab Invest 2003; 83: 1185–1192.
Pabst C, Zustin J, Jacobsen F, Luetkens T, Kroger N, Schilling G et al. Expression and prognostic relevance of MAGE-C1/CT7 and MAGE-C2/CT10 in osteolytic lesions of patients with multiple myeloma. Exp Mol Pathol 2010; 89: 175–181.
Zhuang R, Zhu Y, Fang L, Liu XS, Tian Y, Chen LH et al. Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun 2006; 6: 7.
Yu H, Lee H, Herrmann A, Buettner R, Jove R . Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014; 14: 736–746.
Yu H, Kortylewski M, Pardoll D . Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7: 41–51.
Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 2003; 4: 540–545.
Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol 2003; 4: 546–550.
Zhang X, Guo A, Yu J, Possemato A, Chen Y, Zheng W et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci USA 2007; 104: 4060–4064.
Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science 1997; 278: 1803–1805.
Buettner R, Mora LB, Jove R . Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 2002; 8: 945–954.
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 2001; 20: 2499–2513.
Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007; 117: 3846–3856.
Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001; 28: 29–35.
Brantley EC, Nabors LB, Gillespie GY, Choi YH, Palmer CA, Harrison K et al. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res 2008; 14: 4694–4704.
Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol 2002; 168: 466–474.
Pineda CT, Ramanathan S, Fon Tacer K, Weon JL, Potts MB, Ou YH et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 2015; 160: 715–728.
Su S, Minges JT, Grossman G, Blackwelder AJ, Mohler JL, Wilson EM . Proto-oncogene activity of melanoma antigen-A11 (MAGE-A11) regulates retinoblastoma-related p107 and E2F1 proteins. J Biol Chem 2013; 288: 24809–24824.
Marcar L, Ihrig B, Hourihan J, Bray SE, Quinlan PR, Jordan LB et al. MAGE-A cancer/testis antigens inhibit MDM2 ubiquitylation function and promote increased levels of MDM4. PLoS One 2015; 10: e0127713.
Yang B, O'Herrin SM, Wu J, Reagan-Shaw S, Ma Y, Bhat KM et al. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res 2007; 67: 9954–9962.
Bhatia N, Xiao TZ, Rosenthal KA, Siddiqui IA, Thiyagarajan S, Smart B et al. MAGE-C2 promotes growth and tumorigenicity of melanoma cells, phosphorylation of KAP1, and DNA damage repair. J Invest Dermatol 2013; 133: 759–767.
Sahai E, Garcia-Medina R, Pouyssegur J, Vial E . Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J Cell Biol 2007; 176: 35–42.
Kitzing TM, Wang Y, Pertz O, Copeland JW, Grosse R . Formin-like 2 drives amoeboid invasive cell motility downstream of RhoC. Oncogene 2010; 29: 2441–2448.
Parri M, Taddei ML, Bianchini F, Calorini L, Chiarugi P . EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res 2009; 69: 2072–2081.
Elson-Schwab I, Lorentzen A, Marshall CJ . MicroRNA-200 family members differentially regulate morphological plasticity and mode of melanoma cell invasion. PLoS One 2010; 5: e13176.
Christoph DC, Kasper S, Gauler TC, Loesch C, Engelhard M, Theegarten D et al. BetaV-tubulin expression is associated with outcome following taxane-based chemotherapy in non-small cell lung cancer. Br J Cancer 2012; 107: 823–830.
Acknowledgements
We thank Drs Xinmin Cao (National University of Singapore), Zhijie Chang (Tsinghua University) and Ning Guo (Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences) for providing vectors. This work was supported by grants from National Natural Science Foundation of China (no. 81472645) and Beijing Natural Science Foundation (7142087).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Song, X., Hao, J., Wang, J. et al. The cancer/testis antigen MAGEC2 promotes amoeboid invasion of tumor cells by enhancing STAT3 signaling. Oncogene 36, 1476–1486 (2017). https://doi.org/10.1038/onc.2016.314
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.314