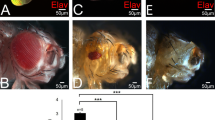
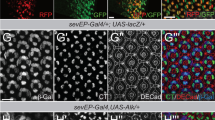
Abstract
The tumor-suppressor RUNX3 has a critical role in a lineage determination, cell cycle arrest and apoptosis. Lozenge (Lz), a Drosophila homolog of mammalian RUNX family members, has integral roles in these processes and specifically in eye cell fate determination. To elucidate the genetic modifiers of Lz/RUNX3, we performed a large-scale functional screen in a fly mutant library. The screen revealed genetic interactions between the Lz, Rac and Hippo pathways. Analysis of interactions among these genes revealed that the defective phenotype resulting from activation of Yki, an end point effector of the Hippo pathway, was suppressed by Lz and enhanced by Rac-Trio. Molecular biological analysis using mammalian homologs reveled that LATS1/2-mediated YAP phosphorylation-facilitated dissociation of the YAP-TEAD4 complex and association of the YAP-RUNX3 complex. When cells were stimulated to proliferate, activated RAC-TRIO signaling inhibited LATS1/2-mediated YAP phosphorylation; consequently, YAP dissociated from RUNX3 and associated with TEAD, thereby replacing the YAP-RUNX3 complex with YAP-TEAD. RUNX3 contributed to both association and dissociation of YAP-TEAD complex, most likely through the formation of the YAP-TEAD-RUNX3 ternary complex. Ectopic expression of RUNX3 in MKN28 gastric cancer cells reduced tumorigenicity, and the tumor-suppressive activity of RUNX3 was associated with its ability to interact with YAP. These results identify a novel regulatory mechanism, mediated by the Hippo and RAC-TRIO pathways, that changes the binding partner of YAP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ito Y, Bae SC, Chuang LS . The RUNX family: developmental regulators in cancer. Nat Rev Cancer 2015; 15: 81–95.
Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 2002; 109: 113–124.
Qiao Y, Lin SJ, Chen Y, Voon DC, Zhu F, Chuang LS et al. RUNX3 is a novel negative regulator of oncogenic TEAD-YAP complex in gastric cancer. Oncogene 2015; 35: 2664–2674.
Lee YS, Bae SC . How do K-RAS-activated cells evade cellular defense mechanisms? Oncogene 2015; 35: 827–832.
Lee YS, Lee JW, Jang JW, Chi XZ, Kim JH, Li YH et al. Runx3 inactivation is a crucial early event in the development of lung adenocarcinoma. Cancer Cell 2013; 24: 603–616.
Canon J, Banerjee U . Runt and Lozenge function in Drosophila development. Semin Cell Dev Biol 2000; 11: 327–336.
Daga A, Karlovich CA, Dumstrei K, Banerjee U . Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev 1996; 10: 1194–1205.
Flores GV, Daga A, Kalhor HR, Banerjee U . Lozenge is expressed in pluripotent precursor cells and patterns multiple cell types in the Drosophila eye through the control of cell-specific transcription factors. Development 1998; 125: 3681–3687.
Pan D . The hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505.
Yu FX, Guan KL . The Hippo pathway: regulators and regulations. Genes Dev 2013; 27: 355–371.
Xu T, Wang W, Zhang S, Stewart RA, Yu W . Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 1995; 121: 1053–1063.
Boggiano JC, Vanderzalm PJ, Fehon RG . Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell 2011; 21: 888–895.
Poon CL, Lin JI, Zhang X, Harvey KF . The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell 2011; 21: 896–906.
Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007; 130: 1120–1133.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21: 2747–2761.
Oh H, Irvine KD . In vivo regulation of Yorkie phosphorylation and localization. Development 2008; 135: 1081–1088.
Ren F, Zhang L, Jiang J . Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev Biol 2010; 337: 303–312.
Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A . SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol 2008; 18: 435–441.
Wu S, Liu Y, Zheng Y, Dong J, Pan D . The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 2008; 14: 388–398.
Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J . The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell 2008; 14: 377–387.
Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML . TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev 2001; 15: 1229–1241.
Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J 2004; 23: 790–799.
Zhao B, Ye X, Yu J, Li L, Li W, Li S et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008; 22: 1962–1971.
Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y . A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J 1999; 18: 2551–2562.
Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 2007; 447: 1017–1020.
Rorth P . A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc Natl Acad Sci USA 1996; 93: 12418–12422.
Wildonger J, Mann RS . The t(8;21) translocation converts AML1 into a constitutive transcriptional repressor. Development 2005; 132: 2263–2272.
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y . Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004; 101: 7618–7623.
Bouquier N, Vignal E, Charrasse S, Weill M, Schmidt S, Leonetti JP et al. A cell active chemical GEF inhibitor selectively targets the Trio/RhoG/Rac1 signaling pathway. Chem Biol 2009; 16: 657–666.
Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol 2012; 19: 955–962.
Adler JJ, Johnson DE, Heller BL, Bringman LR, Ranahan WP, Conwell MD et al. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc Natl Acad Sci USA 2013; 110: 17368–17373.
Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol 2015; 17: 500–510.
Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 2014; 25: 831–845.
Mo JS, Park HW, Guan KL . The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 2014; 15: 642–656.
Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 2014; 16: 357–366.
Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA 2014; 111: E89–E98.
Wada K, Itoga K, Okano T, Yonemura S, Sasaki H . Hippo pathway regulation by cell morphology and stress fibers. Development 2011; 138: 3907–3914.
Levy D, Reuven N, Shaul Y . A regulatory circuit controlling Itch-mediated p73 degradation by Runx. J Biol Chem 2008; 283: 27462–27468.
Acknowledgements
S-CB is supported by a Creative Research Grant (2014R1A3A2030690) through the National Research Foundation (NRF) of Korea and a Research Grant of Chungbuk National University in 2012. J-WJ is supported by Basic Science Research Program (2014R1A1A2009492) through the NRF funded by the Ministry of Education of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jang, JW., Kim, MK., Lee, YS. et al. RAC-LATS1/2 signaling regulates YAP activity by switching between the YAP-binding partners TEAD4 and RUNX3. Oncogene 36, 999–1011 (2017). https://doi.org/10.1038/onc.2016.266
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.266
This article is cited by
-
YAP 5-methylcytosine modification increases its mRNA stability and promotes the transcription of exosome secretion-related genes in lung adenocarcinoma
Cancer Gene Therapy (2023)
-
Aberrant MET activation impairs perinuclear actin cap organization with YAP1 cytosolic relocation
Communications Biology (2023)
-
Thrombospondin 1 and Reelin act through Vldlr to regulate cardiac growth and repair
Basic Research in Cardiology (2023)
-
Molecular regulation of trophoblast stem cell self-renewal and giant cell differentiation by the Hippo components YAP and LATS1
Stem Cell Research & Therapy (2022)
-
Elevated MST1 leads to apoptosis via depletion of YAP1 in cardiomyocytes exposed to high glucose
Molecular Medicine (2021)