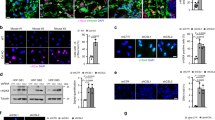
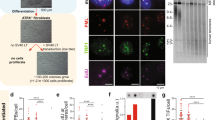
Abstract
Phosphorylation of the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) at the Thr2609 cluster is essential for its complete function in DNA repair and tissue stem cell homeostasis. This phenomenon is demonstrated by congenital bone marrow failure occurring in DNA-PKcs3A/3A mutant mice, which require bone marrow transplantation (BMT) to prevent early mortality. Surprisingly, an increased incidence of spontaneous tumors, especially skin cancer, was observed in adult BMT-rescued DNA-PKcs3A/3A mice. Upon further investigation, we found that spontaneous γH2AX foci occurred in DNA-PKcs3A/3A skin biopsies and primary keratinocytes and that these foci overlapped with telomeres during mitosis, indicating impairment of telomere replication and maturation. Consistently, we observed significantly elevated frequencies of telomere fusion events in DNA-PKcs3A/3A cells as compared with wild-type and DNA-PKcs-knockout cells. In addition, a previously identified DNA-PKcs Thr2609Pro mutation, found in breast cancer, also induces a similar impairment of telomere leading-end maturation. Taken together, our current analyses indicate that the functional DNA-PKcs T2609 cluster is required to facilitate telomere leading strand maturation and prevention of genomic instability and cancer development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Davis AJ, Chen DJ . DNA double strand break repair via non-homologous end-joining. Transl Cancer Res 2013; 2: 130–143.
Chen BP, Uematsu N, Kobayashi J, Lerenthal Y, Krempler A, Yajima H et al. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem 2007; 282: 6582–6587.
Ding Q, Reddy YV, Wang W, Woods T, Douglas P, Ramsden DA et al. Autophosphorylation of the catalytic subunit of the DNA-dependent protein kinase is required for efficient end processing during DNA double-strand break repair. Mol Cell Biol 2003; 23: 5836–5848.
Reddy YV, Ding Q, Lees-Miller SP, Meek K, Ramsden DA . Non-homologous end joining requires that the DNA-PK complex undergo an autophosphorylation-dependent rearrangement at DNA ends. J Biol Chem 2004; 279: 39408–39413.
Chan DW, Chen BP, Prithivirajsingh S, Kurimasa A, Story MD, Qin J et al. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev 2002; 16: 2333–2338.
Meek K, Douglas P, Cui X, Ding Q, Lees-Miller SP . Trans autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining. Mol Cell Biol 2007; 27: 3881–3890.
Yajima H, Lee KJ, Chen BP . ATR-dependent phosphorylation of DNA-dependent protein kinase catalytic subunit in response to UV-induced replication stress. Mol Cell Biol 2006; 26: 7520–7528.
Hammel M, Yu Y, Mahaney BL, Cai B, Ye R, Phipps BM et al. Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial non-homologous end joining complex. J Biol Chem 2010; 285: 1414–1423.
Zhang S, Yajima H, Huynh H, Zheng J, Callen E, Chen HT et al. Congenital bone marrow failure in DNA-PKcs mutant mice associated with deficiencies in DNA repair. J Cell Biol 2011; 193: 295–305.
Mikkola HK, Orkin SH . The journey of developing hematopoietic stem cells. Development 2006; 133: 3733–3744.
Mirchandani KD, D'Andrea AD . The Fanconi anemia/BRCA pathway: a coordinator of cross-link repair. Exp Cell Res 2006; 312: 2647–2653.
McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet 2008; 40: 963–970.
Nishio N, Kojima S . Recent progress in dyskeratosis congenita. Int J Hematol 2010; 92: 419–424.
Kirwan M, Dokal I . Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet 2008; 73: 103–112.
Hockemeyer D, Palm W, Wang RC, Couto SS, de Lange T . Engineered telomere degradation models dyskeratosis congenita. Genes Dev 2008; 22: 1773–1785.
He H, Wang Y, Guo X, Ramchandani S, Ma J, Shen MF et al. Pot1b deletion and telomerase haploinsufficiency in mice initiate an ATR-dependent DNA damage response and elicit phenotypes resembling dyskeratosis congenita. Mol Cell Biol 2009; 29: 229–240.
Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH . Strand-specific postreplicative processing of mammalian telomeres. Science 2001; 293: 2462–2465.
Gilley D, Tanaka H, Hande MP, Kurimasa A, Li GC, Oshimura M et al. DNA-PKcs is critical for telomere capping. Proc Natl Acad Sci USA 2001; 98: 15084–15088.
Goytisolo FA, Samper E, Edmonson S, Taccioli GE, Blasco MA . The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol Cell Biol 2001; 21: 3642–3651.
Jiang W, Crowe JL, Liu X, Nakajima S, Wang Y, Li C et al. Differential phosphorylation of DNA-PKcs regulates the interplay between end-processing and end-ligation during nonhomologous end-joining. Mol Cell 2015; 58: 172–185.
Williams ES, Klingler R, Ponnaiya B, Hardt T, Schrock E, Lees-Miller SP et al. Telomere dysfunction and DNA-PKcs deficiency: characterization and consequence. Cancer Res 2009; 69: 2100–2107.
Ishii-Ohba H, Kobayashi S, Nishimura M, Shimada Y, Tsuji H, Sado T et al. Existence of a threshold-like dose for gamma-ray induction of thymic lymphomas and no susceptibility to radiation-induced solid tumors in SCID mice. Mutat Res 2007; 619: 124–133.
de Lange T . Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19: 2100–2110.
Hultdin M, Gronlund E, Norrback KF, Eriksson-Lindstrom E, Just T, Roos G . Telomere analysis by fluorescence in situ hybridization and flow cytometry. Nucleic Acids Res 1998; 26: 3651–3656.
Laud PR, Multani AS, Bailey SM, Wu L, Ma J, Kingsley C et al. Elevated telomere–telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev 2005; 19: 2560–2570.
Hsu FM, Zhang S, Chen BP . Role of DNA-dependent protein kinase catalytic subunit in cancer development and treatment. Transl Cancer Res 2012; 1: 22–34.
Wang X, Szabo C, Qian C, Amadio PG, Thibodeau SN, Cerhan JR et al. Mutational analysis of thirty-two double-strand DNA break repair genes in breast and pancreatic cancers. Cancer Res 2008; 68: 971–975.
Convery E, Shin EK, Ding Q, Wang W, Douglas P, Davis LS et al. Inhibition of homologous recombination by variants of the catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs). Proc Natl Acad Sci USA 2005; 102: 1345–1350.
Espejel S, Franco S, Sgura A, Gae D, Bailey SM, Taccioli GE et al. Functional interaction between DNA-PKcs and telomerase in telomere length maintenance. EMBO J 2002; 21: 6275–6287.
Espejel S, Martin M, Klatt P, Martin-Caballero J, Flores JM, Blasco MA . Shorter telomeres, accelerated ageing and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep 2004; 5: 503–509.
Ruis BL, Fattah KR, Hendrickson EA . DNA-PKcs regulates proliferation, telomere length and genomic stability in human somatic cells. Mol Cell Biol 2008; 28: 6182–6195.
Chai W, Du Q, Shay JW, Wright WE . Human telomeres have different overhang sizes at leading versus lagging strands. Mol Cell 2006; 21: 427–435.
Chow TT, Zhao Y, Mak SS, Shay JW, Wright WE . Early and late steps in telomere overhang processing in normal human cells: the position of the final RNA primer drives telomere shortening. Genes Dev 2012; 26: 1167–1178.
Zhao Y, Sfeir AJ, Zou Y, Buseman CM, Chow TT, Shay JW et al. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 2009; 138: 463–475.
Wu P, van Overbeek M, Rooney S, de Lange T . Apollo contributes to G overhang maintenance and protects leading-end telomeres. Mol Cell 2010; 39: 606–617.
Verdun RE, Crabbe L, Haggblom C, Karlseder J . Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol Cell 2005; 20: 551–561.
Sui J, Lin YF, Xu K, Lee KJ, Wang D, Chen BP . DNA-PKcs phosphorylates hnRNP-A1 to facilitate the RPA-to-POT1 switch and telomere capping after replication. Nucleic Acids Res 2015; 43: 5971–5983.
Wang Y, Ghosh G, Hendrickson EA . Ku86 represses lethal telomere deletion events in human somatic cells. Proc Natl Acad Sci USA 2009; 106: 12430–12435.
Hayashi MT, Cesare AJ, Fitzpatrick JAJ, Lazzerini-Denchi E, Karlseder J . A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat Struct Mol Biol 2012; 19: 387–394.
Bombarde O, Boby C, Gomez D, Frit P, Giraud-Panis MJ, Gilson E et al. TRF2/RAP1 and DNA-PK mediate a double protection against joining at telomeric ends. EMBO J 2010; 29: 1573–1584.
Khadka P, Lee JH, Baek SH, Oh SY, Chung IK . DNA-PKcs-interacting protein KIP binding to TRF2 is required for the maintenance of functional telomeres. Biochem J 2014; 463: 19–30.
Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JH et al. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol 2004; 2: E240.
Giunta S, Belotserkovskaya R, Jackson SP . DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol 2010; 190: 197–207.
Heijink AM, Krajewska M, van Vugt MA . The DNA damage response during mitosis. Mutat Res 2013; 750: 45–55.
Orthwein A, Fradet-Turcotte A, Noordermeer SM, Canny MD, Brun CM, Strecker J et al. Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science 2014; 344: 189–193.
Yu H, Bauer B, Lipke GK, Phillips RL, Van Zant G . Apoptosis and hematopoiesis in murine fetal liver. Blood 1993; 81: 373–384.
Poon SS, Lansdorp PM . Quantitative fluorescence in situ hybridization (Q-FISH). In: Bonifacino JS, Harford JB, Lippincott-Schwartz J, Yamada KM eds Current Protocols in Cell Biology Chapter 18 John Wiley and Sons Inc.: Hoboken, NJ, USA, 2001, p 14.
Potts PR, Yu H . The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat Struct Mol Biol 2007; 14: 581–590.
Schoenfeld DA . Sample-size formula for the proportional-hazards regression model. Biometrics 1983; 39: 499–503.
Acknowledgements
This work was supported by National Institutes of Health (CA166677) and the Cancer Prevention Research Institute of Texas (RP110465) to BPC. We thank Dr Titia de Lange for kindly providing anti-TRF1 antibody, and Dr Damiana Chiavolini for critical reading and editing of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, S., Matsunaga, S., Lin, YF. et al. Spontaneous tumor development in bone marrow-rescued DNA-PKcs3A/3A mice due to dysfunction of telomere leading strand deprotection. Oncogene 35, 3909–3918 (2016). https://doi.org/10.1038/onc.2015.459
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.459
This article is cited by
-
DNA–dependent protein kinase in telomere maintenance and protection
Cellular & Molecular Biology Letters (2020)
-
DNA-PKcs has KU-dependent function in rRNA processing and haematopoiesis
Nature (2020)
-
ATM, DNA-PKcs and ATR: shaping development through the regulation of the DNA damage responses
Genome Instability & Disease (2020)