Abstract
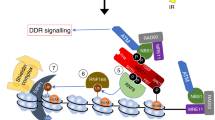
Genome integrity is vital to cellular homeostasis and its forfeiture is linked to deleterious consequences—cancer, immunodeficiency, genetic disorders and premature aging. The human ubiquitin ligase Pso4/Prp19 has emerged as a critical component of multiple DNA damage response (DDR) signaling networks. It not only senses DNA damage, binds double-stranded DNA in a sequence-independent manner, facilitates processing of damaged DNA, promotes DNA end joining, regulates replication protein A (RPA2) phosphorylation and ubiquitination at damaged DNA, but also regulates RNA splicing and mitotic spindle formation in its integral capacity as a scaffold for a multimeric core complex. Accordingly, by virtue of its regulatory and structural interactions with key proteins critical for genome integrity—DNA double-strand break (DSB) repair, DNA interstrand crosslink repair, repair of stalled replication forks and DNA end joining—it fills a unique niche in restoring genomic integrity after multiple types of DNA damage and thus has a vital role in maintaining chromatin integrity and cellular functions. These properties may underlie its ability to thwart replicative senescence and, not surprisingly, have been linked to the self-renewal and colony-forming ability of murine hematopoietic stem cells. This review highlights recent advances in hPso4 research that provides a fascinating glimpse into the pleiotropic activities of a ubiquitously expressed multifunctional E3 ubiquitin ligase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003; 426: 194–198.
von Zglinicki T, Saretzki G, Ladhoff J, d'Adda di Fagagna F, Jackson SP . Human cell senescence as a DNA damage response. Mech Ageing Dev 2005; 126: 111–117.
Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH . DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol 2008; 10: 1241–1247.
Dellago H, Khan A, Nussbacher M, Gstraunthaler A, Lammermann I, Schosserer M et al. ATM-dependent phosphorylation of SNEVhPrp19/hPso4 is involved in extending cellular life span and suppression of apoptosis. Aging 2012; 4: 290–304.
Reinhardt HC, Schumacher B . The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet 2012; 28: 128–136.
Jiang G, Plo I, Wang T, Rahman M, Cho JH, Yang E et al. BRCA1-Ku80 protein interaction enhances end-joining fidelity of chromosomal double-strand breaks in the G1 phase of the cell cycle. J Biol Chem 2013; 288: 8966–8976.
Gupta GP, Vanness K, Barlas A, Manova-Todorova KO, Wen YH, Petrini JH . The Mre11 complex suppresses oncogene-driven breast tumorigenesis and metastasis. Mol Cell 2013; 52: 353–365.
Lee JH, Paull TT . Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 2007; 26: 7741–7748.
Lammens K, Bemeleit DJ, Mockel C, Clausing E, Schele A, Hartung S et al. The Mre11:Rad50 structure shows an ATP-dependent molecular clamp in DNA double-strand break repair. Cell 2011; 145: 54–66.
Lee BS, Gapud EJ, Zhang S, Dorsett Y, Bredemeyer A, George R et al. Functional intersection of ATM and DNA-dependent protein kinase catalytic subunit in coding end joining during V(D)J recombination. Mol Cell Biol 2013; 33: 3568–3579.
Zha S, Jiang W, Fujiwara Y, Patel H, Goff PH, Brush JW et al. Ataxia telangiectasia-mutated protein and DNA-dependent protein kinase have complementary V(D)J recombination functions. Proc Natl Acad Sci USA 2011; 108: 2028–2033.
Perkins EJ, Nair A, Cowley DO, Van Dyke T, Chang Y, Ramsden DA . Sensing of intermediates in V(D)J recombination by ATM. Genes Dev 2002; 16: 159–164.
Zha S, Guo C, Boboila C, Oksenych V, Cheng HL, Zhang Y et al. ATM damage response and XLF repair factor are functionally redundant in joining DNA breaks. Nature 2011; 469: 250–254.
Kakarougkas A, Jeggo PA . DNA DSB repair pathway choice: an orchestrated handover mechanism. Br J Radiol 2014; 87: 20130685.
Motoyama N, Naka K . DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Dev 2004; 14: 11–16.
Howard SM, Yanez DA, Stark JM . DNA damage response factors from diverse pathways, including DNA crosslink repair, mediate alternative end joining. PLoS Genet 2015; 11: e1004943.
Mateos-Gomez PA, Gong F, Nair N, Miller KM, Lazzerini-Denchi E, Sfeir A . Mammalian polymerase theta promotes alternative NHEJ and suppresses recombination. Nature 2015; 518: 254–257.
Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin MI et al. Homologous-recombination-deficient tumours are dependent on Poltheta-mediated repair. Nature 2015; 518: 258–262.
Vermeij WP, Hoeijmakers JH, Pothof J . Aging: not all DNA damage is equal. Curr Opin Genet Dev 2014; 26: 124–130.
Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D et al. DNA polymerase beta deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res 2015; 43: 943–959.
Iyama T, Lee SY, Berquist BR, Gileadi O, Bohr VA, Seidman MM et al. CSB interacts with SNM1A and promotes DNA interstrand crosslink processing. Nucleic Acids Res 2015; 43: 247–258.
Kolodner RD, Putnam CD, Myung K . Maintenance of genome stability in Saccharomyces cerevisiae. Science 2002; 297: 552–557.
Myung K, Kolodner RD . Suppression of genome instability by redundant S-phase checkpoint pathways in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 2002; 99: 4500–4507.
Jaehnig EJ, Kuo D, Hombauer H, Ideker TG, Kolodner RD . Checkpoint kinases regulate a global network of transcription factors in response to DNA damage. Cell Rep 2013; 4: 174–188.
da Silva KV, de Morais Junior MA, Henriques JA . The PSO4 gene of S. cerevisiae is important for sporulation and the meiotic DNA repair of photoactivated psoralen lesions. Curr Genet 1995; 27: 207–212.
Grey M, Dusterhoft A, Henriques JA, Brendel M . Allelism of PSO4 and PRP19 links pre-mRNA processing with recombination and error-prone DNA repair in Saccharomyces cerevisiae. Nucleic Acids Res 1996; 24: 4009–4014.
Gotzmann J, Gerner C, Meissner M, Holzmann K, Grimm R, Mikulits W et al. hNMP 200: a novel human common nuclear matrix protein combining structural and regulatory functions. Exp Cell Res 2000; 261: 166–179.
Mahajan KN, Mitchell BS . Role of human Pso4 in mammalian DNA repair and association with terminal deoxynucleotidyl transferase. Proc Natl Acad Sci USA 2003; 100: 10746–10751.
Grillari J, Ajuh P, Stadler G, Loscher M, Voglauer R, Ernst W et al. SNEV is an evolutionarily conserved splicing factor whose oligomerization is necessary for spliceosome assembly. Nucleic Acids Res 2005; 33: 6868–6883.
Chan SP, Kao DI, Tsai WY, Cheng SC . The Prp19p-associated complex in spliceosome activation. Science 2003; 302: 279–282.
Vander Kooi CW, Ohi MD, Rosenberg JA, Oldham ML, Newcomer ME, Gould KL et al. The Prp19 U-box crystal structure suggests a common dimeric architecture for a class of oligomeric E3 ubiquitin ligases. Biochemistry 2006; 45: 121–130.
Vander Kooi CW, Ren L, Xu P, Ohi MD, Gould KL, Chazin WJ . The Prp19 WD40 domain contains a conserved protein interaction region essential for its function. Structure 2010; 18: 584–593.
Ohi MD, Vander Kooi CW, Rosenberg JA, Ren L, Hirsch JP, Chazin WJ et al. Structural and functional analysis of essential pre-mRNA splicing factor Prp19p. Mol Cell Biol 2005; 25: 451–460.
Aravind L, Iyer LM, Koonin EV . Scores of RINGS but no PHDs in ubiquitin signaling. Cell Cycle 2003; 2: 123–126.
Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL . Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat Struct Biol 2003; 10: 250–255.
Grote M, Wolf E, Will CL, Lemm I, Agafonov DE, Schomburg A et al. Molecular architecture of the human Prp19/CDC5L complex. Mol Cell Biol 2010; 30: 2105–2119.
Tarn WY, Hsu CH, Huang KT, Chen HR, Kao HY, Lee KR et al. Functional association of essential splicing factor(s) with PRP19 in a protein complex. EMBO J 1994; 13: 2421–2431.
Song EJ, Werner SL, Neubauer J, Stegmeier F, Aspden J, Rio D et al. The Prp19 complex and the Usp4Sart3 deubiquitinating enzyme control reversible ubiquitination at the spliceosome. Genes Dev 2010; 24: 1434–1447.
Makarova OV, Makarov EM, Urlaub H, Will CL, Gentzel M, Wilm M et al. A subset of human 35S U5 proteins, including Prp19, function prior to catalytic step 1 of splicing. EMBO J 2004; 23: 2381–2391.
Tarn WY, Lee KR, Cheng SC . Yeast precursor mRNA processing protein PRP19 associates with the spliceosome concomitant with or just after dissociation of U4 small nuclear RNA. Proc Natl Acad Sci USA 1993; 90: 10821–10825.
Chan SP, Cheng SC . The Prp19-associated complex is required for specifying interactions of U5 and U6 with pre-mRNA during spliceosome activation. J Biol Chem 2005; 280: 31190–31199.
David CJ, Boyne AR, Millhouse SR, Manley JL . The RNA polymerase II C-terminal domain promotes splicing activation through recruitment of a U2AF65-Prp19 complex. Genes Dev 2011; 25: 972–983.
Lu X, Legerski RJ . The Prp19/Pso4 core complex undergoes ubiquitylation and structural alterations in response to DNA damage. Biochem Biophys Res Commun 2007; 354: 968–974.
Marin I . Ancient origin of animal U-box ubiquitin ligases. BMC Evol Biol 2010; 10: 331.
Legerski RJ . The Pso4 complex splices into the DNA damage response. Cell Cycle 2009; 8: 3448–3449.
Zhang N, Kaur R, Lu X, Shen X, Li L, Legerski RJ . The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J Biol Chem 2005; 280: 40559–40567.
Beck BD, Park SJ, Lee YJ, Roman Y, Hromas RA, Lee SH . Human Pso4 is a metnase (SETMAR)-binding partner that regulates metnase function in DNA repair. J Biol Chem 2008; 283: 9023–9030.
de Andrade HH, Marques EK, Schenberg AC, Henriques JA . The PSO4 gene is responsible for an error-prone recombinational DNA repair pathway in Saccharomyces cerevisiae. Mol Gen Genet 1989; 217: 419–426.
Abbas M, Shanmugam I, Bsaili M, Hromas R, Shaheen M . The role of the human psoralen 4 (hPso4) protein complex in replication stress and homologous recombination. J Biol Chem 2014; 289: 14009–14019.
Alvarez-Quilon A, Serrano-Benitez A, Lieberman JA, Quintero C, Sanchez-Gutierrez D, Escudero LM et al. ATM specifically mediates repair of double-strand breaks with blocked DNA ends. Nat Commun 2014; 5: 3347.
Lim DS, Kim ST, Xu B, Maser RS, Lin J, Petrini JH et al. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 2000; 404: 613–617.
Paull TT . Mechanisms of ATM Activation. Annu Rev Biochem 2015; 84: 711–738.
Bakkenist CJ, Kastan MB . DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499–506.
Berkovich E, Monnat RJ Jr, Kastan MB . Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat Cell Biol 2007; 9: 683–690.
Kaufmann WK . The human intra-S checkpoint response to UVC-induced DNA damage. Carcinogenesis 2010; 31: 751–765.
Kim ST, Lim DS, Canman CE, Kastan MB . Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem 1999; 274: 37538–37543.
Smolka MB, Albuquerque CP, Chen SH, Zhou H . Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc Natl Acad Sci USA 2007; 104: 10364–10369.
Benjamin AB, Zhou X, Isaac O, Zhao H, Song Y, Chi X et al. PRP19 upregulation inhibits cell proliferation in lung adenocarcinomas by p21-mediated induction of cell cycle arrest. Biomed Pharmacother 2014; 68: 463–470.
Kuo PC, Tsao YP, Chang HW, Chen PH, Huang CW, Lin ST et al. Breast cancer amplified sequence 2, a novel negative regulator of the p53 tumor suppressor. Cancer Res 2009; 69: 8877–8885.
Zhang N, Kaur R, Akhter S, Legerski RJ . Cdc5L interacts with ATR and is required for the S-phase cell-cycle checkpoint. EMBO Rep 2009; 10: 1029–1035.
Zhang J, Dewar JM, Budzowska M, Motnenko A, Cohn MA, Walter JC . DNA interstrand cross-link repair requires replication-fork convergence. Nat Struct Mol Biol 2015; 22: 242–247.
Wan L, Huang J . The PSO4 protein complex associates with replication protein A (RPA) and modulates the activation of ataxia telangiectasia-mutated and Rad3-related (ATR). J Biol Chem 2014; 289: 6619–6626.
Marechal A, Li JM, Ji XY, Wu CS, Yazinski SA, Nguyen HD et al. PRP19 transforms into a sensor of RPA-ssDNA after DNA damage and drives ATR activation via a ubiquitin-mediated circuitry. Mol Cell 2014; 53: 235–246.
Rodriguez M, Yu X, Chen J, Songyang Z . Phosphopeptide binding specificities of BRCA1 COOH-terminal (BRCT) domains. J Biol Chem 2003; 278: 52914–52918.
Koonin EV, Altschul SF, Bork P . BRCA1 protein products…Functional motifs. Nat Genet 1996; 13: 266–268.
Yu X, Chini CC, He M, Mer G, Chen J . The BRCT domain is a phospho-protein binding domain. Science 2003; 302: 639–642.
Woods NT, Mesquita RD, Sweet M, Carvalho MA, Li X, Liu Y et al. Charting the landscape of tandem BRCT domain-mediated protein interactions. Sci Signal 2012; 5: rs6.
Mahajan KN, Gangi-Peterson L, Sorscher DH, Wang J, Gathy KN, Mahajan NP et al. Association of terminal deoxynucleotidyl transferase with Ku. Proc Natl Acad Sci USA 1999; 96: 13926–13931.
Gangi-Peterson L, Sorscher DH, Reynolds JW, Kepler TB, Mitchell BS . Nucleotide pool imbalance and adenosine deaminase deficiency induce alterations of N-region insertions during V(D)J recombination. J Clin Invest 1999; 103: 833–841.
Purugganan MM, Shah S, Kearney JF, Roth DB . Ku80 is required for addition of N nucleotides to V(D)J recombination junctions by terminal deoxynucleotidyl transferase. Nucleic Acids Res 2001; 29: 1638–1646.
Bennardo N, Cheng A, Huang N, Stark JM . Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet 2008; 4: e1000110.
Tobin LA, Robert C, Rapoport AP, Gojo I, Baer MR, Tomkinson AE et al. Targeting abnormal DNA double-strand break repair in tyrosine kinase inhibitor-resistant chronic myeloid leukemias. Oncogene 2013; 32: 1784–1793.
Cordaux R, Udit S, Batzer MA, Feschotte C . Birth of a chimeric primate gene by capture of the transposase gene from a mobile element. Proc Natl Acad Sci USA 2006; 103: 8101–8106.
Liu D, Bischerour J, Siddique A, Buisine N, Bigot Y, Chalmers R . The human SETMAR protein preserves most of the activities of the ancestral Hsmar1 transposase. Mol Cell Biol 2007; 27: 1125–1132.
Roman Y, Oshige M, Lee YJ, Goodwin K, Georgiadis MM, Hromas RA et al. Biochemical characterization of a SET and transposase fusion protein, Metnase: its DNA binding and DNA cleavage activity. Biochemistry 2007; 46: 11369–11376.
Wray J, Williamson EA, Royce M, Shaheen M, Beck BD, Lee SH et al. Metnase mediates resistance to topoisomerase II inhibitors in breast cancer cells. PLoS One 2009; 4: e5323.
Williamson EA, Rasila KK, Corwin LK, Wray J, Beck BD, Severns V et al. The SET and transposase domain protein Metnase enhances chromosome decatenation: regulation by automethylation. Nucleic Acids Res 2008; 36: 5822–5831.
Beck BD, Lee SS, Williamson E, Hromas RA, Lee SH . Biochemical characterization of metnase's endonuclease activity and its role in NHEJ repair. Biochemistry 2011; 50: 4360–4370.
Beck BD, Lee SS, Hromas R, Lee SH . Regulation of Metnase's TIR binding activity by its binding partner, Pso4. Arch Biochem Biophys 2010; 498: 89–94.
Kuraoka I, Ito S, Wada T, Hayashida M, Lee L, Saijo M et al. Isolation of XAB2 complex involved in pre-mRNA splicing, transcription, and transcription-coupled repair. J Biol Chem 2008; 283: 940–950.
Chan YA, Hieter P, Stirling PC . Mechanisms of genome instability induced by RNA-processing defects. Trends Genet 2014; 30: 245–253.
Tresini M, Warmerdam DO, Kolovos P, Snijder L, Vrouwe MG, Demmers JA et al. The core spliceosome as target and effector of non-canonical ATM signalling. Nature 2015; 523: 53–58.
Henriques JA, Vicente EJ, Leandro da Silva KV, Schenberg AC . PSO4: a novel gene involved in error-prone repair in Saccharomyces cerevisiae. Mut Res 1989; 218: 111–124.
Fortschegger K, Wagner B, Voglauer R, Katinger H, Sibilia M, Grillari J . Early embryonic lethality of mice lacking the essential protein SNEV. Mol Cell Biol 2007; 27: 3123–3130.
Schraml E, Voglauer R, Fortschegger K, Sibilia M, Stelzer I, Grillari J et al. Haploinsufficiency of senescence evasion factor causes defects of hematopoietic stem cells functions. Stem Cells Dev 2008; 17: 355–366.
Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL . Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 2007; 447: 725–729.
Hofmann JC, Tegha-Dunghu J, Drager S, Will CL, Luhrmann R, Gruss OJ . The Prp19 complex directly functions in mitotic spindle assembly. PLoS One 2013; 8: e74851.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI . U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem 2001; 276: 33111–33120.
Meyer C, Hofmann J, Burmeister T, Groger D, Park TS, Emerenciano M et al. The MLL recombinome of acute leukemias in 2013. Leukemia 2013; 27: 2165–2176.
Kleinridders A, Pogoda HM, Irlenbusch S, Smyth N, Koncz C, Hammerschmidt M et al. PLRG1 is an essential regulator of cell proliferation and apoptosis during vertebrate development and tissue homeostasis. Mol Cell Biol 2009; 29: 3173–3185.
Grillari J, Loscher M, Denegri M, Lee K, Fortschegger K, Eisenhaber F et al. Blom7alpha is a novel heterogeneous nuclear ribonucleoprotein K homology domain protein involved in pre-mRNA splicing that interacts with SNEVPrp19-Pso4. J Biol Chem 2009; 284: 29193–29204.
Loscher M, Fortschegger K, Ritter G, Wostry M, Voglauer R, Schmid JA et al. Interaction of U-box E3 ligase SNEV with PSMB4, the beta7 subunit of the 20S proteasome. Biochem J 2005; 388: 593–603.
Dellago H, Loscher M, Ajuh P, Ryder U, Kaisermayer C, Grillari-Voglauer R et al. Exo70, a subunit of the exocyst complex, interacts with SNEV(hPrp19/hPso4) and is involved in pre-mRNA splicing. Biochem J 2011; 438: 81–91.
Acknowledgements
I thank Dr Nupam Mahajan and Professor William Kaufmann (UNC Chapel Hill) for critical reading of the manuscript and suggestions. I appreciate Urvashi Mahajan for help with the illustrations. This work is supported in part by Department of Defense Awards W81XWH-12-1-0248, W81XWH-14-1-0251 and W81XWH-15-1-0059 to KM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Mahajan, K. hPso4/hPrp19: a critical component of DNA repair and DNA damage checkpoint complexes. Oncogene 35, 2279–2286 (2016). https://doi.org/10.1038/onc.2015.321
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.321
This article is cited by
-
PRPF19 functions in DNA damage repair and gemcitabine sensitivity via regulating DDB1 in bladder cancer cells
Cytotechnology (2024)
-
PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9
Cell Death & Disease (2023)
-
Genetic alterations of the SUMO isopeptidase SENP6 drive lymphomagenesis and genetic instability in diffuse large B-cell lymphoma
Nature Communications (2022)
-
Interactome of vertebrate GAF/ThPOK reveals its diverse functions in gene regulation and DNA repair
Journal of Biosciences (2020)