Abstract
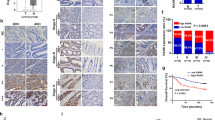
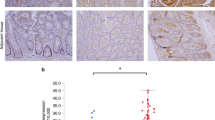
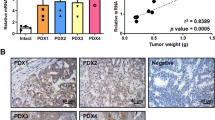
Tumor metastasis is the major cause of death among cancer patients, with >90% of cancer-related death attributable to the spreading of metastatic cells to secondary organs. Store-operated Ca2+ entry (SOCE) is the predominant Ca2+ entry mechanism in most cancer cells, and stromal interaction molecule 1 (STIM1) is the endoplasmic reticulum (ER) Ca2+ sensor for store-operated channels. Here we reported that the STIM1 was overexpressed in colorectal cancer (CRC) patients. STIM1 overexpression in CRC was significantly associated with tumor size, depth of invasion, lymph node metastasis status and serum levels of carcinoembryonic antigen. Furthermore, ectopic expression of STIM1 promoted CRC cell motility, while depletion of STIM1 with short hairpin RNA inhibited CRC cell migration. Our data further suggested that STIM1 promoted CRC cell migration through increasing the expression of cyclooxygenase-2 (COX-2) and production of prostaglandin E2 (PGE2). Importantly, ectopically expressed COX-2 or exogenous PGE2 were able to rescue migration defect in STIM1 knockdown CRC cells, and inhibition of COX-2 with ibuprofen and indomethacin abrogated STIM1-mediated CRC cell motility. In short, our data provided clinicopathological significance for STIM1 and SOCE in CRC progression, and implicated a role for COX-2 in STIM1-mediated CRC metastasis. Our studies also suggested a new approach to inhibit STIM1-mediated metastasis with COX-2 inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schulz RA, Yutzey KE . Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol 2004; 266: 1–16.
Yang S, Zhang JJ, Huang XY . Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009; 15: 124–134.
Prevarskaya N, Skryma R, Shuba Y . Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer 2011; 11: 609–618.
Yang S, Huang XY . Ca2+ Influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem 2005; 280: 27130–27137.
Berridge MJ, Lipp P, Bootman MD . The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1: 11–21.
Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005; 169: 435–445.
Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 2005; 15: 1235–1241.
Brandman O, Liou J, Park WS, Meyer T . STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007; 131: 1327–1339.
Hogan PG, Lewis RS, Rao A . Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol 2010; 28: 491–533.
Hou MF, Kuo HC, Li JH, Wang YS, Chang CC, Chen KC et al. Orai1/CRACM1 overexpression suppresses cell proliferation via attenuation of the store-operated calcium influx-mediated signalling pathway in A549 lung cancer cells. Biochim Biophys Acta 2011; 1810: 1278–1284.
Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA 2011; 108: 15225–15230.
Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D . Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis 2012; 33: 740–750.
Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D et al. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochem Biophys Res Commun 2011; 411: 786–791.
Jans R, Mottram L, Johnson DL, Brown AM, Sikkink S, Ross K et al. Lysophosphatidic acid promotes cell migration through STIM1- and Orai1-mediated Ca2+(i) mobilization and NFAT2 activation. J Invest Dermatol 2013; 133: 793–802.
Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA et al. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. Faseb J 2009; 23: 2425–2437.
Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell 2010; 143: 84–98.
Parkin DM, Bray F, Ferlay J, Pisani P . Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108.
Wolmark N, Fisher B, Wieand HS, Henry RS, Lerner H, Legault-Poisson S et al. The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials. Ann Surg 1984; 199: 375–382.
McGarrigle D, Shan D, Yang S, Huang XY . Role of tyrosine kinase Csk in G protein-coupled receptor- and receptor tyrosine kinase-induced fibroblast cell migration. J Biol Chem 2006; 281: 10583–10588.
Wang D, Dubois RN . The role of anti-inflammatory drugs in colorectal cancer. Annu Rev Med 2012; 64: 131–144.
Wang JY, Chen BK, Wang YS, Tsai YT, Chen WC, Chang WC et al. Involvement of store-operated calcium signaling in EGF-mediated COX-2 gene activation in cancer cells. Cell Signal 2012; 24: 162–169.
Huang WC, Chai CY, Chen WC, Hou MF, Wang YS, Chiu YC et al. Histamine regulates cyclooxygenase 2 gene activation through Orai1-mediated NFkappaB activation in lung cancer cells. Cell Calcium 2011; 50: 27–35.
Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y et al. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett 2012; 330: 163–169.
Yoshida J, Iwabuchi K, Matsui T, Ishibashi T, Masuoka T, Nishio M . Knockdown of stromal interaction molecule 1 (STIM1) suppresses store-operated calcium entry, cell proliferation and tumorigenicity in human epidermoid carcinoma A431 cells. Biochem Pharmacol 2012; 84: 1592–1603.
Aytes A, Mollevi DG, Martinez-Iniesta M, Nadal M, Vidal A, Morales A et al. $Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol Carcinog 2012; 51: 746–753.
McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther 2011; 10: 448–460.
Shin DH, Seo EY, Pang B, Nam JH, Kim HS, Kim WK et al. Inhibition of Ca2+-release-activated Ca2+ channel (CRAC) and K+ channels by curcumin in Jurkat-T cells. J Pharmacol Sci 2011; 115: 144–154.
Munoz E, Valero RA, Quintana A, Hoth M, Nunez L, Villalobos C . Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/orai channels normally prevented by mitochondria. J Biol Chem 2011; 286: 16186–16196.
Berry PA, Birnie R, Droop AP, Maitland NJ, Collins AT . The calcium sensor STIM1 is regulated by androgens in prostate stromal cells. Prostate 2011; 71: 1646–1655.
Ryu S, McDonnell K, Choi H, Gao D, Hahn M, Joshi N et al. Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer Cell 2013; 23: 63–76.
Chantome A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Gueguinou M et al. Pivotal role of the lipid raftcomplex in human cancer cell migration and bone metastases SK3-Orai1 complex in human cancer cell migration and bone metastases. Cancer Res 2013; 73: 4852–4861.
Sun J, He H, Xiong Y, Lu S, Shen J, Cheng A et al. Fascin protein is critical for transforming growth factor beta protein-induced invasion and filopodia formation in spindle-shaped tumor cells. J Biol Chem 2011; 286: 38865–38875.
Chen L, Yang S, Jakoncic J, Zhang JJ, Huang XY . Migrastatin analogues target fascin to block tumour metastasis. Nature 2010; 464: 1062–1066.
Yang IH, Tsai YT, Chiu SJ, Liu LT, Lee HH, Hou MF et al. Involvement of STIM1 and Orai1 in EGF-mediated cell growth in retinal pigment epithelial cells. J Biomed Sci 2013; 20: 41.
Feng Z, Wang L, Rajski SR, Xu Z, Coeffet-Legal MF, Shen B . Engineered production of iso-migrastatin in heterologous Streptomyces hosts. Bioorg Med Chem 2008; 17: 2147–2153.
Yang S, Zhang JJ, Huang XY . Mouse models for tumor metastasis. Methods Mol Biol 2012; 928: 221–228.
Sun J, He H, Pillai S, Xiong Y, Challa S, Xu L et al. GATA3 transcription factor abrogates Smad4-mediated fascin overexpression, invadopodium formation and breast cancer cell invasion. J Biol Chem 2013; 288: 36971–36982.
Hsu WL, Tsai MH, Lin MW, Chiu YC, Lu JH, Chang CH et al. Differential effects of arsenic on calcium signaling in primary keratinocytes and malignant (HSC-1) cells. Cell Calcium 2012; 52: 161–169.
Acknowledgements
We thank Dr Minjung Kim for assistance with IHC staining and we are grateful to Professor Yun Yen (City of Hope National Medical Center) for reading this paper and providing comments. This study was partly supported by funding from Health and welfare surcharge of tobacco products, Taiwan (Grant no. DOH102-TD-C-111-002), National Science Council, Taiwan (Grant no. NSC98-2320-B-037-028; NSC101-2320-B-038-029-MY3) and Center for Biomarkers and Biotech Drugs, Kaohsiung Medical Universit, Aim for the Top Universities Grant (KMU-TP103C00, KMU-TP103C03) WCC; and an NIH grant R01CA175741 to SY.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, JY., Sun, J., Huang, MY. et al. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene 34, 4358–4367 (2015). https://doi.org/10.1038/onc.2014.366
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.366
This article is cited by
-
STIM1 in tumor cell death: angel or devil?
Cell Death Discovery (2023)
-
Blockage of Orai1-Nucleolin interaction meditated calcium influx attenuates breast cancer cells growth
Oncogenesis (2022)
-
PKC-β modulates Ca2+ mobilization through Stim1 phosphorylation
Genes & Genomics (2022)
-
Connecting Calcium-Based Nanomaterials and Cancer: From Diagnosis to Therapy
Nano-Micro Letters (2022)
-
RANK promotes colorectal cancer migration and invasion by activating the Ca2+-calcineurin/NFATC1-ACP5 axis
Cell Death & Disease (2021)