Abstract
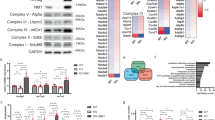
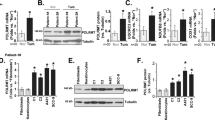
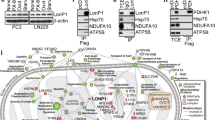
Manganese superoxide dismutase (MnSOD) is a mitochondrially localized primary antioxidant enzyme, known to be essential for the survival of aerobic life and to have important roles in tumorigenesis. Here, we show that MnSOD deficiency in skin tissues of MnSOD-heterozygous knockout (Sod2+/−) mice leads to increased expresson of uncoupling proteins (UCPs). When MnSOD is deficient, superoxide radical and its resulting reactive oxygen species (ROS) activate ligand binding to peroxisome proliferator-activated receptor alpha (PPARα), suggesting that the activation of PPARα signaling is a major mechanism underlying MnSOD-dependent UCPs expression that consequently triggers the PI3K/Akt/mTOR pathway, leading to increased aerobic glycolysis. Knockdown of UCPs and mTOR suppresses lactate production and increases ATP levels, suggesting that UCPs contribute to increased glycolysis. These results highlight the existence of a free radical-mediated mechanism that activates mitochondria uncoupling to reduce ROS production, which precedes the glycolytic adaptation described as the Warburg Effect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Oberley LW, Buettner GR . Role of superoxide dismutase in cancer: a review. Cancer Res 1979; 39: 1141–1149.
Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 1995; 11: 376–381.
Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ et al. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol 2001; 281: H1422–H1432.
Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci 1998; 18: 687–697.
Nagano Y, Matsui H, Shimokawa O, Hirayama A, Tamura M, Nakamura Y et al. Rebamipide attenuates nonsteroidal anti-inflammatory drugs (NSAID) induced lipid peroxidation by the manganese superoxide dismutase (MnSOD) overexpression in gastrointestinal epithelial cells. J Physiol Pharmacol 2012; 63: 137–142.
Kiningham KK, Oberley TD, Lin S, Mattingly CA, St Clair DK . Overexpression of manganese superoxide dismutase protects against mitochondrial-initiated poly(ADP-ribose) polymerase-mediated cell death. FASEB J 1999; 13: 1601–1610.
Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R et al. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol 2001; 24: 436–441.
Miriyala S, Holley AK, St Clair DK . Mitochondrial superoxide dismutase—signals of distinction. Anticancer Agents Med Chem 2011; 11: 181–190.
Holley AK, Dhar SK, St Clair DK . Curbing cancer's sweet tooth: is there a role for MnSOD in regulation of the Warburg effect?. Mitochondrion 2013; 13: 170–188.
Zhao Y, Xue Y, Oberley TD, Kiningham KK, Lin SM, Yen HC et al. Overexpression of manganese superoxide dismutase suppresses tumor formation by modulation of activator protein-1 signaling in a multistage skin carcinogenesis model. Cancer Res 2001; 61: 6082–6088.
Oberley TD, Xue Y, Zhao Y, Kiningham K, Szweda LI, St Clair DK . In situ reduction of oxidative damage, increased cell turnover, and delay of mitochondrial injury by overexpression of manganese superoxide dismutase in a multistage skin carcinogenesis model. Antioxid Redox Signal 2004; 6: 537–548.
Dhar SK, Tangpong J, Chaiswing L, Oberley TD, St Clair DK . Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res 2011; 71: 6684–6695.
Ferreira LM . Cancer metabolism: the Warburg effect today. Exp Mol Pathol 2010; 89: 372–380.
Lin J, Wang J, Greisinger AJ, Grossman HB, Forman MR, Dinney CP et al. Energy balance, the PI3K-AKT-mTOR pathway genes, and the risk of bladder cancer. Cancer Prev Res 2010; 3: 505–517.
Song MS, Salmena L, Pandolfi PP . The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 2012; 13: 283–296.
Miller DM, Thomas SD, Islam A, Muench D, Sedoris K . c-Myc and cancer metabolism. Clin Cancer Res 2012; 18: 5546–5553.
Jarmuszkiewicz W, Woyda-Ploszczyca A, Antos-Krzeminska N, Sluse FE . Mitochondrial uncoupling proteins in unicellular eukaryotes. Biochim Biophys Acta 2010; 1797: 792–799.
Klingenberg M . Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem Sci 1990; 15: 108–112.
Porter RK . Mitochondrial proton leak: a role for uncoupling proteins 2 and 3?. Biochim Biophys Acta 2001; 1504: 120–127.
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002; 415: 96–99.
Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD et al. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res 2005; 65: 3745–3750.
Holley AK, Xu Y, Noel T, Bakthavatchalu V, Batinic-Haberle I, St Clair DK . Manganese superoxide dismutase-mediated inside-out signaling in HaCaT human keratinocytes and SKH-1 mouse skin. Antioxid Redox Signal 2014; 20: 2347–2360.
Sears IB, MacGinnitie MA, Kovacs LG, Graves RA . Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol Cell Biol 1996; 16: 3410–3419.
Aubert J, Champigny O, Saint-Marc P, Negrel R, Collins S, Ricquier D et al. Up-regulation of UCP-2 gene expression by PPAR agonists in preadipose and adipose cells. Biochem Biophys Res Commun 1997; 238: 606–611.
Forman BM, Chen J, Evans RM . Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 1997; 94: 4312–4317.
Jansen S, Cashman K, Thompson JG, Pantaleon M, Kaye PL . Glucose deprivation, oxidative stress and peroxisome proliferator-activated receptor-alpha (PPARA) cause peroxisome proliferation in preimplantation mouse embryos. Reproduction 2009; 138: 493–505.
Teruel T, Hernandez R, Benito M, Lorenzo M . Rosiglitazone and retinoic acid induce uncoupling protein-1 (UCP-1) in a p38 mitogen-activated protein kinase-dependent manner in fetal primary brown adipocytes. J Biol Chem 2003; 278: 263–269.
Oberley LW, Oberley TD . Role of antioxidant enzymes in cell immortalization and transformation. Mol Cell Biochem 1988; 84: 147–153.
Finkel T . Signal transduction by reactive oxygen species. J Cell Biol 2011; 194: 7–15.
Ray PD, Huang BW, Tsuji Y . Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012; 24: 981–990.
Boss O, Hagen T, Lowell BB . Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes 2000; 49: 143–156.
Silva JP, Shabalina IG, Dufour E, Petrovic N, Backlund EC, Hultenby K et al. SOD2 overexpression: enhanced mitochondrial tolerance but absence of effect on UCP activity. EMBO J 2005; 24: 4061–4070.
Echtay KS . Mitochondrial uncoupling proteins—what is their physiological role?. Free Radical Biol Med 2007; 43: 1351–1371.
Sluse FE, Jarmuszkiewicz W, Navet R, Douette P, Mathy G, Sluse-Goffart CM . Mitochondrial UCPs: new insights into regulation and impact. Biochim Biophys Acta 2006; 1757: 480–485.
Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ et al. Polyenephosphatidylcholine prevents alcoholic liver disease in PPAR alpha-null mice through attenuation of increases in oxidative stress. J Hepatol 2009; 50: 1236–1246.
Patterson AD, Shah YM, Matsubara T, Krausz KW, Gonzalez FJ . Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 2012; 56: 281–290.
Baffy G . Uncoupling protein-2 and cancer. Mitochondrion 2010; 10: 243–252.
Valle A, Oliver J, Roca P . Role of uncoupling proteins in cancer. Cancers 2010; 2: 567–591.
Hong Y, Fink BD, Dillon JS, Sivitz WI . Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology 2001; 142: 249–256.
Horimoto M, Resnick MB, Konkin TA, Routhier J, Wands JR, Baffy G . Expression of uncoupling protein-2 in human colon cancer. Clin Cancer Res 2004; 10: 6203–6207.
Joseph JW, Koshkin V, Saleh MC, Sivitz WI, Zhang CY, Lowell BB et al. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem 2004; 279: 51049–51056.
Diano S, Horvath TL . Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med 2012; 18: 52–58.
Samudio I, Fiegl M, McQueen T, Clise-Dwyer K, Andreeff M . The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res 2008; 68: 5198–5205.
Bouillaud F . UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta 2009; 1787: 377–383.
Criscuolo F, Mozo J, Hurtaud C, Nubel T, Bouillaud F . UCP2, UCP3, avUCP, what do they do when proton transport is not stimulated? Possible relevance to pyruvate and glutamine metabolism. Biochim Biophys Acta 2006; 1757: 1284–1291.
Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 2008; 22: 9–18.
Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA 2014; 111: 960–965.
Mathupala SP, Ko YH, Pedersen PL . The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim Biophys Acta 2010; 1797: 1225–1230.
Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A . Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys 1999; 363: 91–97.
Xu Y, Fang F, Miriyala S, Crooks PA, Oberley TD, Chaiswing L et al. KEAP1 is a redox sensitive target that arbitrates the opposing radiosensitive effects of parthenolide in normal and cancer cells. Cancer Res 2013; 73: 4406–4417.
Acknowledgements
This work was supported by National Institutes of Health grants CA 049797 and CA 143428, and an Edward P. Evans Foundation grant to Daret K. St Clair; CA 073599 and CA 143428 to William H. St Clair; and National Nature Science Foundation of China grant 81372199 to Yong Xu. We would like to thank Dr Ronald M. Evans, Howard Hughes Medical Institute, San Diego, California 92186, USA, for providing the PPREx3-TK-luciferase construct for this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, Y., Miriyala, S., Fang, F. et al. Manganese superoxide dismutase deficiency triggers mitochondrial uncoupling and the Warburg effect. Oncogene 34, 4229–4237 (2015). https://doi.org/10.1038/onc.2014.355
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.355