Abstract
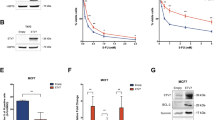
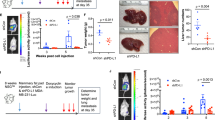
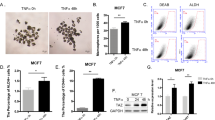
Tumor-initiating cells (TICs) are a sub-population of cells that exhibit a robust ability to self-renew and contribute to the formation of primary tumors, the relapse of previously treated tumors and the development of metastases. TICs have been identified in various tumors including those of the breast, and are particularly enriched in the basal-like and claudin-low subtypes of breast cancer. The signaling pathways that contribute to the function and maintenance of TICs are under intense study. We explored the potential involvement of the nuclear factor-κB (NF-κB) family of transcription factors in TICs in cell lines that are representative of basal-like and claudin-low breast cancer. NF-κB was found to be activated in breast cancer cells that form tumorspheres efficiently. Moreover, both canonical and non-canonical NF-κB signaling is required for these cells to self-renew in vitro and to form xenograft tumors efficiently in vivo using limiting dilutions of cells. Consistent with this fact, canonical and non-canonical NF-κB signaling is activated in TICs isolated from breast cancer cell lines. Experimental results indicate that NF-κB promotes the function of TICs by stimulating epithelial-to-mesenchymal transition and by upregulating the expression of the inflammatory cytokines interleukin-1β and interleukin-6. The results suggest the use of NF-κB inhibitors for clinical therapy of certain breast cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- EMT:
-
epithelial-to-mesenchymal transition
- IκB:
-
inhibitor of κB
- IKK:
-
IκB kinase
- IL:
-
interleukin
- NF-κB:
-
nuclear factor-κB
- TIC:
-
tumor-initiating cell
- TGFβ:
-
transforming growth factor-β
References
Reya T, Morrison SJ, Clarke MF, Weissman IL . Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105–111.
Charafe-Jauffret E, Ginestier C, Birnbaum D . Breast cancer stem cells: tools and models to rely on. BMC Cancer 2009; 9: 202.
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005; 65: 5506–5511.
Croker AK, Allan AL . Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med 2008; 2: 374–390.
Lawson JC, Blatch GL, Edkins AL . Cancer stem cells in breast cancer and metastasis. Breast Cancer Res Treat 2009; 2: 241–254.
Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H . Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 2005; 3: 1154–1159.
Phillips TM, McBride WH, Pajonk F . The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 2006; 24: 1777–1785.
Hambardzumyan D, Squatrito M, Holland EC . Radiation resistance and stem-like cells in brain tumors. Cancer Cell 2006; 6: 454–456.
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008; 9: 672–679.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 2009; 33: 13820–13825.
Lee HE, Kim JH, Kim YJ, Choi SY, Kim SW, Kang E et al. An increase in cancer stem cell population after primary systemic therapy is a poor prognostic factor in breast cancer. Br J Cancer 2011; 11: 1730–1738.
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009; 4: 1302–1313.
Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res 2008; 10: R53.
Nakshatri H, Srour EF, Badve S . Breast cancer stem cells and intrinsic subtypes: controversies rage on. Curr Stem Cell Res Ther 2009; 1: 50–60.
Ghosh S, Karin M . Missing pieces in the NF-kappaB puzzle. Cell 2002; 109: 81–96.
Hayden MS, Ghosh S . Signaling to NF-kappaB. Genes Dev. 2004; 18: 2195–2224.
Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T et al. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol 2004; 10: 6336–6344.
Basseres DS, Baldwin AS . Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 2006; 51: 6817–6830.
Karin M . Nuclear factor-kappaB in cancer development and progression. Nature 2006; 7092: 431–436.
Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS . Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene 2000; 9: 1123–1131.
Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest 1997; 12: 2952–2960.
Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Sledge GW . Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 1997; 7: 3629–3639.
Nakshatri H, Goulet RJ . NF-kappaB and breast cancer. Curr Probl Cancer 2002; 5: 282–309.
Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA 2004; 27: 10137–10142.
Cao Y, Luo JL, Karin M . IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci USA 2007; 40: 15852–15857.
Liu M, Sakamaki T, Casimiro MC, Willmarth NE, Quong AA, Ju X et al. The canonical NF-kappaB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res 2010; 24: 10464–10473.
Yamaguchi N, Ito T, Azuma S, Ito E, Honma R, Yanagisawa Y et al. Constitutive activation of nuclear factor-kappaB is preferentially involved in the proliferation of basal-like subtype breast cancer cell lines. Cancer Sci 2009; 9: 1668–1674.
Pastrana E, Silva-Vargas V, Doetsch F . Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011; 5: 486–498.
Iliopoulos D, Hirsch HA, Struhl K . An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009; 4: 693–706.
Ziegelbauer K, Gantner F, Lukacs NW, Berlin A, Fuchikami K, Niki T et al. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol 2005; 2: 178–192.
Prat A, Perou CM . Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011; 1: 5–23.
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 2010; 12: R68.
Merkhofer EC, Cogswell P, Baldwin AS . Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene 2010; 8: 1238–1248.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003; 7: 3983–3988.
Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004; 14: 4966–4971.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 4: 704–715.
Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A . Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 2008; 3: e2888.
Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 2006; 8: R59.
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S . Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009; 11: R46.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 5: 555–567.
Korkaya H, Paulson A, Iovino F, Wicha MS . HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008; 47: 6120–6130.
Fillmore CM, Kuperwasser C . Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 2008; 10: R25.
Storci G, Sansone P, Mari S, D'Uva G, Tavolari S, Guarnieri T et al. TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol 2010; 3: 682–691.
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ . TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001; 6844: 346–351.
Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK et al. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFkappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem 2008; 36: 24497–24505.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 6: 1420–1428.
Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 2004; 4: 569–581.
Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 2010; 2: 485–497.
Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 2011; 2: 614–624.
Fillmore C, Kuperwasser C . Human breast cancer stem cell markers CD44 and CD24: enriching for cells with functional properties in mice or in man? Breast Cancer Res 2007; 9: 303.
Rajasekhar VK, Studer L, Gerald W, Socci ND, Scher HI . Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat Commun 2011; 2: 162.
Bhat-Nakshatri P, Appaiah H, Ballas C, Pick-Franke P, Goulet R, Badve S et al. SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24− phenotype. BMC Cancer 2010; 10: 411.
Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y . NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat 2008; 3: 419–427.
Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009; 33: 2940–2947.
Wilson W, Baldwin AS . Maintenance of constitutive IkappaB kinase activity by glycogen synthase kinase-3alpha/beta in pancreatic cancer. Cancer Res 2008; 19: 8156–8163.
Steinbrecher KA, Wilson W, Cogswell PC, Baldwin AS . Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol 2005; 19: 8444–8455.
Acknowledgements
We would like to thank members of the Baldwin lab for helpful discussions and comments. MFK and JWB were supported by a T32 National Research Service Award (5-T32-CA009156-37) awarded to the Lineberger Comprehensive Cancer Center at the University of North Carolina at Chapel Hill. MFK was also supported by an F32 National Research Service Award (1F32CA153439-01) from the National Institutes of Health and a post-doctoral Fellowship from the American Cancer Society (120296-PF-11-093-01-DDC). JWB was also supported by an F32 National Research Service Award (1F32CA162628-01) from the National Institutes of Health. Research support was provided by National Cancer Institute, grants CA138937 and CA73756, and by Department of Defense grant BC073048. Additional research support was provided by the Samuel Waxman Cancer Research Foundation.
Author contributions: Dr MFK performed experiments and wrote the manuscript. Dr JWB performed key experiments and edited the manuscript. Dr CLL performed key experiments. KSC performed the tumor xenograft studies. Dr ASB edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Kendellen, M., Bradford, J., Lawrence, C. et al. Canonical and non-canonical NF-κB signaling promotes breast cancer tumor-initiating cells. Oncogene 33, 1297–1305 (2014). https://doi.org/10.1038/onc.2013.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.64
Keywords
This article is cited by
-
Targeting NF-κB in Hepatic Ischemia–Reperfusion Alleviation: from Signaling Networks to Therapeutic Targeting
Molecular Neurobiology (2023)
-
Elevated EDAR signalling promotes mammary gland tumourigenesis with squamous metaplasia
Oncogene (2022)
-
Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance
Cell Death & Differentiation (2022)
-
Modification of BRCA1-associated breast cancer risk by HMMR overexpression
Nature Communications (2022)
-
New uracil analog U-332 is an inhibitor of NF-κB in 5-fluorouracil-resistant human leukemia HL-60 cell line
BMC Pharmacology and Toxicology (2020)