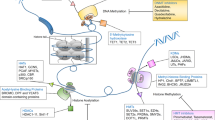
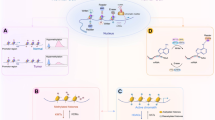
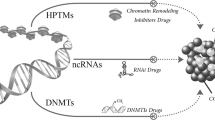
Abstract
Cancer is characterized by uncontrolled cell growth and the acquisition of metastatic properties. In most cases, the activation of oncogenes and/or deactivation of tumour suppressor genes lead to uncontrolled cell cycle progression and inactivation of apoptotic mechanisms. Although the underlying mechanisms of carcinogenesis remain unknown, increasing evidence links aberrant regulation of methylation to tumourigenesis. In addition to the methylation of DNA and histones, methylation of nonhistone proteins, such as transcription factors, is also implicated in the biology and development of cancer. Because the metabolic cycling of methionine is a key pathway for many of these methylating reactions, strategies to target the epigenetic machinery of cancer cells could result in novel and efficient anticancer therapies. The application of these new epigenetic therapies could be of utility in the promotion of E2F1-dependent apoptosis in cancer cells, in avoiding metastatic pathways and/or in sensitizing tumour cells to radiotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cellarier E, Durando X, Vasson MP, Farges MC, Demiden A, Maurizis JC et al. Methionine dependency and cancer treatment. Cancer Treat Rev 2003; 29: 489–499.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Ibáñez C, Simó C, García-Cañas V, Gómez-Martínez A, Ferragut JA, Cifuentes A . CE/LC-MS multiplatform for broad metabolomic analysis of dietary polyphenols effect on colon cancer cells proliferation. Electrophoresis 2012; 33: 2328–2336.
Johnson C, Warmoes MO, Shen X, Locasale JW . Epigenetics and cancer metabolism. Cancer Lett 2013; S0304-3835: 00715–00715.
Yun J, Johnson JL, Hanigan CL, Locasale JW . Interactions between epigenetics and metabolism in cancers. Front Oncol 2012; 2: 163.
Locasale JW . Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 2013; 13: 572–583.
Mund C, Lyko F . Epigenetic cancer therapy: proof of concept and remaining challenges. Bioessays 2010; 32: 949–957.
Limm K, Ott C, Wallner S, Mueller DW, Oefner P, Hellerbrand C et al. Deregulation of protein methylation in melanoma. Eur J Cancer 2013; 49: 1305–1313.
Ushijima T, Okochi-Takada E . Aberrant methylations in cancer cells: where do they come from? Cancer Sci 2005; 96: 206–211.
Versteeg R . Aberrant methylation in cancer. Am J Hum Genet 1997; 60: 751–754.
Anderson ME . Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 1998; 111-112: 1–14.
Thomas T, Thomas TJ . Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci 2001; 58: 244–258.
Ulrey CL, Liu L, Andrews LG, Tollefsbol TO . The impact of metabolism on DNA methylation. Hum Mol Genet 2005; 1: R139–R147.
Hoffman DR, Marion DW, Cornatzer WE, Duerre JA . S-Adenosylmethionine and S-adenosylhomocystein metabolism in isolated rat liver. Effects of L-methionine, L-homocystein, and adenosine. J Biol Chem 1980; 255: 10822–10827.
Robertson KD . DNA methylation, methyltransferases, and cancer. Oncogene 2001; 20: 3139–3155.
Das PM, Singal R . DNA methylation and cancer. J Clin Oncol 2004; 22: 4632–4642.
Widschwendter M, Jones PA . DNA methylation and breast carcinogenesis. Oncogene 2002; 21: 5462–5482.
Esteller M . Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol 2005; 45: 629–656.
He Y, Korboukh I, Jin J, Huang J . Targeting protein lysine methylation and demethylation in cancers. Acta Biochim Biophys Sin 2012; 44: 70–79.
Copeland RA, Solomon ME, Richon VM . Protein methyltransferases as a target class for drug discovery. Nat Rev Drug Discov 2009; 8: 724–732.
Lee JA, Pallas DC . Leucine carboxyl methyltransferase-1 is necessary for normal progression through mitosis in mammalian cells. J Biol Chem 2007; 282: 30974–30984.
Dai Q, Choy E, Chiu V, Romano J, Slivka SR, Steitz SA et al. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J Biol Chem 1998; 273: 15030–15034.
Lee J, Chen Y, Tolstykh T, Stock J . A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc Natl Acad Sci USA 1996; 93: 6043–6047.
Volker C, Stock JB . Carboxyl methylation of Ras-related proteins. Methods Enzymol 1995; 255: 65–82.
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004; 119: 941–953.
Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y . Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell 2005; 19: 857–864.
Prokhortchouk E, Hendrich B . Methyl-CpG binding proteins and cancer: are MeCpGs more important than MBDs? Oncogene 2002; 21: 5394–5399.
Epigenetics Szyf M . DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol 2009; 49: 243–263.
Ng HH, Bird A . Histone deacetylases: silencers for hire. Trends Biochemi Sci 2000; 25: 121–126.
Issa JPJ . DNA methylation as a therapeutic target in cancer. Clin Cancer Res 2007; 13: 1634–1637.
Probst AV, Dunleavy E, Almouzni G . Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol 2009; 10: 192–206.
Li E, Bestor TH, Jaenisch R . Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 1992; 69: 915–926.
Jin B, Li Y, Robertson KD . DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011; 2: 607–617.
Kouzarides T . Chromatin modifications and their function. Cell 2007; 128: 693–705.
Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 2000; 406: 593–599.
Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev 2002; 16: 1779–1791.
Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell 2002; 10: 1107–1117.
Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002; 298: 1039–1043.
Strahl BD, Grant PA, Briggs SD, Sun ZW, Bone JR, Caldwell JA et al. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol Cell Biol 2002; 22: 1298–1306.
Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P et al. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol Cell 2001; 8: 1207–1217.
Wakeman TP, Wang Q, Feng J, Wang XF . Bat3 facilitates H3K79 dimethylation by DOT1L and promotes DNA damage-induced 53BP1 foci at G1/G2 cell-cycle phases. EMBO J 2012; 31: 2169–2181.
Pei H, Zhang L, Luo K, Qin Y, Chesi M, Fei F et al. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature 2011; 470: 124–128.
Nishioka K, Rice JC, Sarma K, Erdjument-Bromage H, Werner J, Wang Y et al. PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell 2002; 9: 1201–1213.
Jørgensen S, Elvers I, Trelle MB, Menzel T, Eskildsen M, Jensen ON et al. The histone methyltransferase SET8 is required for S-phase progression. J Cell Biol 2007; 179: 1337–1345.
Fang J, Feng Q, Ketel CS, Wang H, Cao R, Xia L et al. Purification and functional characterization of SET8, a nucleosomal histone H4-lysine 20-specific methyltransferase. Curr Biol 2002; 12: 1086–1099.
Huyen Y, Zgheib O, Ditullio RA Jr, Gorgoulis VG, Zacharatos P, Petty TJ et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 2004; 432: 406–411.
Surova O, Zhivotovsky B . Various modes of cell death induced by DNA damage. Oncogene 2013; 32: 3789–3797.
Schulze JM, Jackson J, Nakanishi S, Gardner JM, Hentrich T, Haug J et al. Linking cell cycle to histone modifications: SBF and H2B monoubiquitination machinery and cell-cycle regulation of H3K79 dimethylation. Mol Cell 2009; 35: 626–641.
Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest 2012; 122: 1469–1486.
Dong C, Wu Y, Wang Y, Wang C, Kang T, Rychahou PG et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene 2013; 32: 1351–1362.
Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS et al. Regulation of p53 activity through lysine methylation. Nature 2004; 432: 353–360.
Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA et al. Repression of p53 activity by Smyd2-mediated methylation. Nature 2006; 444: 629–632.
Shi X, Kachirskaia I, Yamaguchi H, West LE, Wen H, Wang EW et al. Modulation of p53 function by SET8-mediated methylation at lysine 382. Mol Cell 2007; 27: 636–646.
Huang J, Dorsey J, Chuikov S, Pérez-Burgos L, Zhang X, Jenuwein T et al. G9a and Glp methylate lysine 373 in the tumor suppressor p53. J Biol Chem 2010; 285: 9636–9641.
Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M et al. p53 is regulated by the lysine demethylase LSD1. Nature 2007; 449: 105–108.
Bell LA, Ryan KM . Life and death decisions by E2F1. Cell Death Differ 2004; 11: 137–142.
Kontaki H, Talianidis I . Lysine methylation regulates E2F1-induced cell death. Mol Cell 2010; 39: 152–160.
Kontaki H, Talianidis I . Cross-talk between post-translational modifications regulate life or death decisions by E2F1. Cell Cycle 2010; 9: 3836–3837.
Xie Q, Bai Y, Wu J, Sun Y, Wang Y, Zhang Y et al. Methylation-mediated regulation of E2F1 in DNA damage-induced cell death. J Recept Signal Transduct Res 2011; 31: 139–146.
Martínez-Balbás MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T . Regulation of E2F1 activity by acetylation. EMBO J 2000; 19: 662–671.
Urist M, Tanaka T, Poyurovsky MV, Prives C . p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev 2004; 18: 3041–3054.
Lin WC, Lin FT, Nevins JR . Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev 2001; 15: 1833–1844.
Kurash JK, Lei H, Shen Q, Marston WL, Granda BW, Fan H et al. Methylation of p53 by Set7/9 mediates p53 acetylation and activity in vivo. Mol Cell 2008; 29: 392–400.
Wang A, Li CJ, Reddy PV, Pardee AB . Cancer chemotherapy by deoxynucleotide depletion and E2F-1 elevation. Cancer Res 2005; 65: 7809–7814.
Pardee AB, Li CJ, Reddy GP . Regulation in S phase by E2F. Cell Cycle 2004; 3: 1091–1094.
Fernández-Pérez MP, Montenegro MF, Sáez-Ayala M, Sánchez-del-Campo L, Piñero-Madrona A, Cabezas-Herrera J et al. Suppression of antifolate resistance by targeting the myosin Va trafficking pathway in melanoma. Neoplasia 2013; 15: 826–839.
Sáez-Ayala M, Montenegro MF, Sánchez-Del-Campo L, Fernández-Pérez MP, Chazarra S, Freter R et al. Directed phenotype switching as an effective antimelanoma strategy. Cancer Cell 2013; 24: 105–119.
Montenegro MF, Sáez-Ayala M, Piñero-Madrona A, Cabezas-Herrera J, Rodríguez-López JN . Reactivation of the tumour suppressor RASSF1A in breast cancer by simultaneous targeting of DNA and E2F1 methylation. PLoS One 2012; 7: e52231.
Sánchez-del-Campo L, Rodríguez-López JN . Targeting the methionine cycle for melanoma therapy with 3-O-(3,4,5-trimethoxybenzoyl)-(−)-epicatechin. Int J Cancer 2008; 123: 2446–2455.
Kishi T, Tanaka Y, Ueda K . Evidence for hypomethylation in two children with acute lymphoblastic leukemia and leukoencephalopathy. Cancer 2000; 89: 925–931.
Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K et al. S-adenosylmethionine and methylation. FASEB J 1996; 10: 471–480.
Winter-Vann AM, Kamen BA, Bergo MO, Young SG, Melnyk S, James SJ et al. Targeting Ras signaling through inhibition of carboxyl methylation: an unexpected property of methotrexate. Proc Natl Acad Sci USA 2003; 100: 6529–6534.
Philips MR . Methotrexate and Ras methylation: a new trick for an old drug? Sci STKE 2004; 2004: pe13.
Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG . Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem 2000; 275: 17605–17610.
Saez-Ayala M, Fernandez-Perez M, Montenegro MF, Sánchez-del-Campo L, Chazarra S, Piñero-Madrona A et al. Melanoma coordinates general and cell-specific mechanisms to promote methotrexate resistance. Exp Cell Res 2012; 318: 1146–1159.
Kufe DW, Wick MM, Abelson HT . Natural resistance to methotrexate in human melanomas. J Invest Dermatol 1980; 75: 357–359.
Sánchez-del-Campo L, Montenegro MF, Cabezas-Herrera J, Rodríguez-López JN . The critical role of alpha-folate receptor in the resistance of melanoma to methotrexate. Pigment Cell Melanoma Res 2009; 22: 588–600.
Chen KG, Valencia JC, Lai B, Zhang G, Paterson JK, Rouzaud F et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci USA 2006; 103: 9903–9907.
Xie T, Nguyen T, Hupe M, Wei ML . Multidrug resistance decreases with mutations of melanosomal regulatory genes. Cancer Res 2009; 69: 992–999.
Huang ZM, Chinen M, Chang PJ, Xie T, Zhong L, Demetriou S et al. Targeting protein-trafficking pathways alters melanoma treatment sensitivity. Proc Natl Acad Sci USA 2012; 109: 553–558.
Navarro-Perán E, Cabezas-Herrera J, García-Cánovas F, Durrant MC, Thorneley RN, Rodríguez-López JN . The antifolate activity of tea catechins. Cancer Res 2005; 65: 2059–2064.
Navarro-Perán E, Cabezas-Herrera J, Hiner AN, Sadunishvili T, García-Cánovas F, Rodríguez-López JN . Kinetics of the inhibition of bovine liver dihydrofolate reductase by tea catechins: origin of slow-binding inhibition and pH studies. Biochemistry 2005; 44: 7512–7525.
Navarro-Martínez MD, Navarro-Perán E, Cabezas-Herrera J, Ruiz-Gómez J, García-Cánovas F, Rodríguez-López JN . Antifolate activity of epigallocatechin gallate against Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2005; 49: 2914–2920.
Navarro-Martínez MD, García-Cánovas F, Rodríguez-López JN . Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. J Antimicrob Chemother 2006; 57: 1083–1092.
Spina M, Cuccioloni M, Mozzicafreddo M, Montecchia F, Pucciarelli S, Eleuteri AM et al. Mechanism of inhibition of wt-dihydrofolate reductase from E. coli by tea epigallocatechin-gallate. Proteins 2008; 72: 240–251.
Hannewald P, Maunit B, Muller JF . Screening of DHFR-binding drugs by MALDI-TOFMS. Anal Bioanal Chem 2008; 392: 1335–1344.
Kao TT, Wang KC, Chang WN, Lin CY, Chen BH, Wu HL et al. Characterization and comparative studies of zebrafish and human recombinant dihydrofolate reductases—inhibition by folic acid and polyphenols. Drug Metab Dispos 2008; 36: 508–516.
Navarro-Perán E, Cabezas-Herrera J, Campo LS, Rodríguez-López JN . Effects of folate cycle disruption by the green tea polyphenol epigallocatechin-3-gallate. Int J Biochem Cell Biol 2007; 39: 2215–2225.
Alemdaroglu NC, Wolffram S, Boissel JP, Closs E, Spahn-Langguth H, Langguth P . Inhibition of folic acid uptake by catechins and tea extracts in Caco-2 cells. Planta Med 2007; 73: 27–32.
Matsuzaki M, Haruna M, Ota E, Sasaki S, Nagai Y, Murashima S . Dietary folate intake, use of folate supplements, lifestyle factors, and serum folate levels among pregnant women in Tokyo, Japan. J Obstet Gynaecol Res 2008; 34: 971–979.
Shiraishi M, Haruna M, Matsuzaki M, Ota E, Murayama R, Murashima S . Association between the serum folate levels and tea consumption during pregnancy. Biosci Trends 2010; 4: 225–230.
Sánchez-del-Campo L, Otón F, Tárraga A, Cabezas-Herrera J, Chazarra S, Rodríguez-López JN . Synthesis and biological activity of a 3,4,5-trimethoxybenzoyl ester analogue of epicatechin-3-gallate. J Med Chem 2008; 51: 2018–2026.
Sánchez-del-Campo L, Tárraga A, Montenegro MF, Cabezas-Herrera J, Rodríguez-López JN . Melanoma activation of 3-O-(3,4,5-trimethoxybenzoyl)-(−)-epicatechin to a potent irreversible inhibitor of dihydrofolate reductase. Mol Pharm 2009; 6: 883–894.
Sánchez-del-Campo L, Chazarra S, Montenegro MF, Cabezas-Herrera J, Rodríguez-López JN . Mechanism of dihydrofolate reductase downregulation in melanoma by 3-O-(3,4,5-trimethoxybenzoyl)-(−)-epicatechin. J Cell Biochem 2010; 110: 1399–1409.
Fidler IJ . Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res 1978; 38: 2651–2660.
Arnheiter H . The discovery of the microphthalmia locus and its gene, Mitf. Pigment Cell Melanoma Res 2010; 23: 729–735.
Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 1993; 74: 395–404.
Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev 2006; 20: 3426–3439.
Hoek K, Goding CR . Cancer stem cells versus phenotype switching in melanoma. Pigment Cell Melanoma Res 2010; 23: 746–759.
Cheli Y, Guiliano S, Botton T, Rocchi S, Hofman V, Hofman P et al. Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene 2011; 30: 2307–2318.
Goodall J, Carreira S, Denat L, Kobi D, Davidson I, Nuciforo P et al. Brn-2 represses microphthalmia-associated transcription factor expression and marks a distinct subpopulation of microphthalmia-associated transcription factor-negative melanoma cells. Cancer Res 2008; 68: 7788–7794.
Kobi D, Steunou AL, Dembele D, Legras S, Larue L, Nieto L et al. Genome-wide analysis of POU3F2/BRN2 promoter occupancy in human melanoma cells reveals Kitl as a novel regulated target gene. Pigment Cell Melanoma Res 2010; 23: 404–418.
Carreira S, Goodall J, Aksan I, La Rocca SA, Galibert MD, Denat L et al. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 2005; 433: 764–769.
Cheli Y, Ohanna M, Ballotti R, Bertolotto C . Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res 2010; 23: 27–40.
Loercher AE, Tank EM, Delston RB, Harbour JW . MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J Cell Biol 2005; 168: 35–40.
Sáez-Ayala M, Sánchez-del-Campo L, Montenegro MF, Chazarra S, Tárraga A, Cabezas-Herrera J et al. Comparison of a pair of synthetic tea-catechin-derived epimers: synthesis, antifolate activity, and tyrosinase-mediated activation in melanoma. ChemMedChem 2011; 6: 440–449.
Spano D, Marshall JC, Marino N, De Martino D, Romano A, Scoppettuolo MN et al. Dipyridamole prevents triple-negative breast-cancer progression. Clin Exp Metastasis 2013; 30: 47–68.
Donninger H, Vos MD, Clark GJ . The RASSF1A tumor suppressor. J Cell Sci 2007; 120: 3163–3172.
Kawamoto K, Okino ST, Place RF, Urakami S, Hirata H, Kikuno N et al. Epigenetic modifications of RASSF1A gene through chromatin remodeling in prostate cancer. Clin Cancer Res 2007; 13: 2541–2548.
Yan PS, Shi H, Rahmatpanah F, Hsiau TH, Hsiau AH, Leu YW et al. Differential distribution of DNA methylation within the RASSF1A CpG island in breast cancer. Cancer Res 2003; 63: 6178–6186.
Jiang Y, Cui L, Chen W, Shen S, Ding L . The prognostic role of RASSF1A promoter methylation in breast cancer: a meta-analysis of published data. PLoS One 2012; 7: e36780.
Jenal M, Trinh E, Britschgi C, Britschgi A, Roh V, Vorburger SA et al. The tumor suppressor gene hypermethylated in cancer 1 is transcriptionally regulated by E2F1. Mol Cancer Res 2009; 7: 916–922.
Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 2007; 27: 962–975.
Guo C, Zhang X, Pfeifer GP . The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J Biol Chem 2011; 286: 6253–6261.
Shi Y . Serine/threonine phosphatases: mechanism through structure. Cell 2009; 139: 468–484.
Acknowledgements
This work was supported by grants from the Ministerio de Ciencia e Innovación (MICINN) (SAF2009-12043-C02-01) and Fundación Séneca (FS) (15230/PI/10). MFM is contracted by the Fundación de la Asociación Española contra el Cáncer (FAECC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Montenegro, M., Sánchez-del-Campo, L., Fernández-Pérez, M. et al. Targeting the epigenetic machinery of cancer cells. Oncogene 34, 135–143 (2015). https://doi.org/10.1038/onc.2013.605
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.605
Keywords
This article is cited by
-
Targeting protein methylation in pancreatic cancer cells results in KRAS signaling imbalance and inhibition of autophagy
Cell Death & Disease (2023)
-
Discovery of FNDR-20123, a histone deacetylase inhibitor for the treatment of Plasmodium falciparum malaria
Malaria Journal (2020)
-
Identification of the metabolic alterations associated with the multidrug resistant phenotype in cancer and their intercellular transfer mediated by extracellular vesicles
Scientific Reports (2017)
-
Oroxylin a could be a Promising Radiosensitizer for Esophageal Squamous Cell Carcinoma by Inducing G2/M Arrest and Activating Apoptosis
Pathology & Oncology Research (2017)
-
Targeting the epigenetics of the DNA damage response in breast cancer
Cell Death & Disease (2016)