Abstract
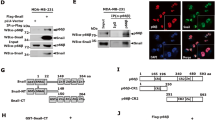
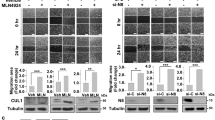
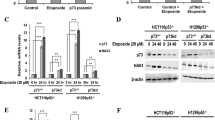
High mobility group box 1 (HMGB1) is a 25-kDa chromatin-associated protein that aids in transcription and DNA repair by directly binding to DNA and altering its conformation. Additionally, HMGB1 can act as an extracellular ligand. When released from dying or stressed cells, HMGB1 binds to the RAGE receptor and activates the p42/44 MAP kinase (MAPK) cascade. HMGB1 is overexpressed in many types of cancer and frequently associated with tumor stage and metastasis. This has predominantly been attributed to an autocrine function that drives MAPK pathway activity. However, by using tumor cells with activating MAPK pathway mutations, we have identified a role for HMGB1 in promoting metastasis and tumor growth that is independent of this pathway. In the absence of HMGB1, these tumor cells show defective in vitro migration as well as reduced metastasis and tumor growth in vivo despite high p42/44 phosphorylation. We found that semaphorin 3A (SEMA3A), previously shown to act as a suppressor of angiogenesis and migration, was highly increased during expression in the absence of HMGB1. SEMA3A/HMGB1 double knockdown rescued the migration defect in HMGB1 single knockdown cells. HMGB1 bound at the semaphorin 3A genomic locus, promoted hetrochromatin formation, and decreased occupancy of acetylated histones. Based on human tumor gene expression databases, HMGB1 was significantly inversely correlated with SEMA3A, suggesting that this mechanism may be more widely relevant in different cancer types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res 2007; 13: 2836–2848.
Volp K, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D et al. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut 2006; 55: 234–242.
Maeda S, Hikiba Y, Shibata W, Ohmae T, Yanai A, Ogura K et al. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem Biophys Res Commun 2007; 360: 394–400.
Cheng BQ, Jia CQ, Liu CT, Lu XF, Zhong N, Zhang ZL et al. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis 2008; 40: 446–452.
Wu D, Ding Y, Wang S, Zhang Q, Liu L . Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol 2008; 216: 167–175.
Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H . Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate 2005; 64: 92–100.
Poser I, Golob M, Buettner R, Bosserhoff AK . Upregulation of HMG1 leads to melanoma inhibitory activity expression in malignant melanoma cells and contributes to their malignancy phenotype. Mol Cell Biol 2003; 23: 2991–2998.
Brezniceanu ML, Volp K, Bosser S, Solbach C, Lichter P, Joos S et al. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J 2003; 17: 1295–1297.
Malarkey CS, Churchill ME . The high mobility group box: the ultimate utility player of a cell. Trends Biochem Sci 2012; 37: 553–562.
Joshi SR, Sarpong YC, Peterson RC, Scovell WM . Nucleosome dynamics: HMGB1 relaxes canonical nucleosome structure to facilitate estrogen receptor binding. Nucleic Acids Res 2012; 40: 10161–10171.
Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet 1999; 22: 276–280.
Agresti A, Lupo R, Bianchi ME, Muller S . HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun 2003; 302: 421–426.
Jayaraman L, Moorthy NC, Murthy KG, Manley JL, Bustin M, Prives C . High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev 1998; 12: 462–472.
Ge H, Roeder RG . The high mobility group protein HMG1 can reversibly inhibit class II gene transcription by interaction with the TATA-binding protein. J Biol Chem 1994; 269: 17136–17140.
van Beijnum JR, Buurman WA, Griffioen AW . Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008; 11: 91–99.
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000; 405: 354–360.
Liang X, Chavez AR, Schapiro NE, Loughran P, Thorne SH, Amoscato AA et al. Ethyl pyruvate administration inhibits hepatic tumor growth. J Leukoc Biol 2009; 86: 599–607.
Dave SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol 2009; 86: 633–643.
Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 2008; 205: 275–285.
Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, O'Meara S et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther 2006; 5: 2606–2612.
Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G . Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol 2010; 136: 677–684.
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009; 459: 1005–1009.
Lin Q, Yang XP, Fang D, Ren X, Zhou H, Fang J et al. High-mobility group box-1 mediates toll-like receptor 4-dependent angiogenesis. Arterioscler Thromb Vasc Biol 2011; 31: 1024–1032.
Neufeld G, Kessler O . The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 2008; 8: 632–645.
Staton CA, Shaw LA, Valluru M, Hoh L, Koay I, Cross SS et al. Expression of class 3 semaphorins and their receptors in human breast neoplasia. Histopathology 2011; 59: 274–282.
Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A et al. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler Thromb Vasc Biol 2011; 31: 741–749.
Schmidt EF, Strittmatter SM . The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol 2007; 600: 1–11.
Schmidt EF, Shim SO, Strittmatter SM . Release of MICAL autoinhibition by semaphorin-plexin signaling promotes interaction with collapsin response mediator protein. J Neurosci 2008; 28: 2287–2297.
Ranzato E, Patrone M, Pedrazzi M, Burlando B . Hmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys 2010; 57: 9–17.
McCauley MJ, Zimmerman J, Maher LJ 3rd, Williams MC . HMGB binding to DNA: single and double box motifs. J Mol Biol 2007; 374: 993–1004.
El Gazzar M, Yoza BK, Chen X, Garcia BA, Young NL, McCall CE . Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance. Mol Cell Biol 2009; 29: 1959–1971.
Sapojnikova N, Maman J, Myers FA, Thorne AW, Vorobyev VI, Crane-Robinson C . Biochemical observation of the rapid mobility of nuclear HMGB1. Biochim Biophys Acta 2005; 1729: 57–63.
Paquette J, Tokuyasu T . EGAN: exploratory gene association networks. Bioinformatics 2010; 26: 285–286.
Szak ST, Mays D, Pietenpol JA . Kinetics of p53 binding to promoter sites in vivo. Mol Cell Biol 2001; 21: 3375–3386.
Acknowledgements
We thank the UCSF Tetrad program for providing funding. We thank Dr Lewis Lanier for all of his advice. We thank Dr Byron Hann, Dr Paul Phojanakong and the UCSF Clinical Therapeutics core for all of their help with mouse xenograft experiments and the UCSF Genome core for their help with gene expression studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nehil, M., Paquette, J., Tokuyasu, T. et al. High mobility group box 1 promotes tumor cell migration through epigenetic silencing of semaphorin 3A. Oncogene 33, 5151–5162 (2014). https://doi.org/10.1038/onc.2013.459
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.459
Keywords
This article is cited by
-
HMGB1/SET/HAT1 complex-mediated SASH1 repression drives glycolysis and metastasis in lung adenocarcinoma
Oncogene (2023)
-
Role of high-mobility group box 1 in methamphetamine-induced activation and migration of astrocytes
Journal of Neuroinflammation (2015)