Abstract
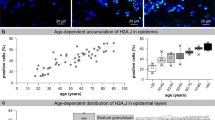
The incidence of skin cancer is increasing worldwide and cutaneous squamous cell carcinomas (SCCs) are associated with considerable morbidity and mortality, particularly in immunosuppressed individuals (‘carcinomatous catastrophy’). Yet, molecular mechanisms are still insufficiently understood. Besides ultraviolet (UV)-indicative mutations, chromosomal aberrations are prominent. As telomeres are essential in preserving chromosome integrity, and telomere erosion as well as aberrant spatial telomere distribution contribute to genomic instability, we first established telomere length profiles across the whole tissue and identified normal skin (10/30) harboring discrete epidermal sites (stem cell territories) of evenly short telomeres. Precancerous actinic keratoses (AKs) (17) and SCCs (27) expressed two telomere phenotypes: (i) tissue-wide evenly short to intermediate and (ii) longer and tissue-wide heterogeneous telomere lengths, suggesting two modes of initiation, with one likely to originate in the epidermal stem cells. Although tumor histotype, location, patient gender or age failed to distinguish the two SCC telomere phenotypes, as did telomerase activity, we found a trend for a higher degree of aberrant p53 and cyclin D1 expression with long/heterogeneous telomeres. In addition, we established an association for the short/homogeneous telomeres with a simpler and the heterogeneous telomeres with a more complex karyotype correlating also with distinct chromosomal changes. SCCs (13) from renal transplant recipients displayed the same telomere dichotomy, suggesting that both telomere subtypes contribute to ‘carcinomatous catastrophy’ under immunosuppression by selecting for a common set (3, 9p and 17q) and subtype-specific aberrations (e.g., 6p gain, 13q loss). As a second mechanism of telomere-dependent genomic instability, we investigated changes in telomere distribution with its most severe form of telomeric aggregates (TAs). We identified a telomere length-independent but progression-dependent increase in cells with small telomere associations in AKs (17/17) and additional TAs in SCCs (24/32), basal cell carcinomas (30/31) and malignant melanomas (15/15), and provide evidence for a reactive oxygen species-dependent mechanism in this UV-induced telomere organization-dependent genomic instability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Euvrard S, Kanitakis J, Claudy A . Skin cancers after organ transplantation. N Engl J Med 2003; 348: 1681–1691.
de Fijter JW . Use of proliferation signal inhibitors in non-melanoma skin cancer following renal transplantation. Nephrol Dial Transplant 2007; 22 (Suppl 1): i23–i26.
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363: 809–819.
Boukamp P . Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis 2005; 26: 1657–1667.
Burnworth B, Arendt S, Muffler S, Steinkraus V, Brocker EB, Birek C et al. The multi-step process of human skin carcinogenesis: a role for p53, cyclin D1, hTERT, p16, and TSP-1. Eur J Cell Biol 2007; 86: 763–780.
Pacifico A, Goldberg LH, Peris K, Chimenti S, Leone G, Ananthaswamy HN . Loss of CDKN2A and p14ARF expression occurs frequently in human nonmelanoma skin cancers. Br J Dermatol 2008; 158: 291–297.
Kanellou P, Zaravinos A, Zioga M, Stratigos A, Baritaki S, Soufla G et al. Genomic instability, mutations and expression analysis of the tumour suppressor genes p14(ARF), p15(INK4b), p16(INK4a) and p53 in actinic keratosis. Cancer Lett 2008; 264: 145–161.
Burnworth B, Popp S, Stark HJ, Steinkraus V, Brocker EB, Hartschuh W et al. Gain of 11q/cyclin D1 overexpression is an essential early step in skin cancer development and causes abnormal tissue organization and differentiation. Oncogene 2006; 25: 4399–4412.
Utikal J, Udart M, Leiter U, Kaskel P, Peter RU, Krahn G . Numerical abnormalities of the Cyclin D1 gene locus on chromosome 11q13 in non-melanoma skin cancer. Cancer Lett 2005; 219: 197–204.
Toll A, Salgado R, Yebenes M, Martin-Ezquerra G, Gilaberte M, Baro T et al. MYC gene numerical aberrations in actinic keratosis and cutaneous squamous cell carcinoma. Br J Dermatol 2009; 161: 1112–1118.
Jin Y, Martins C, Jin C, Salemark L, Jonsson N, Persson B et al. Nonrandom karyotypic features in squamous cell carcinomas of the skin. Genes Chromosomes Cancer 1999; 26: 295–303.
Popp S, Waltering S, Herbst C, Moll I, Boukamp P . UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer 2002; 99: 352–360.
Purdie KJ, Lambert SR, Teh MT, Chaplin T, Molloy G, Raghavan M et al. Allelic imbalances and microdeletions affecting the PTPRD gene in cutaneous squamous cell carcinomas detected using single nucleotide polymorphism microarray analysis. Genes Chromosomes Cancer 2007; 46: 661–669.
Purdie KJ, Harwood CA, Gulati A, Chaplin T, Lambert SR, Cerio R et al. Single nucleotide polymorphism array analysis defines a specific genetic fingerprint for well-differentiated cutaneous SCCs. J Invest Dermatol 2009; 129: 1562–1568.
Salgado R, Toll A, Espinet B, Gonzalez-Roca E, Barranco CL, Serrano S et al. Analysis of cytogenetic abnormalities in squamous cell carcinoma by array comparative genomic hybridization. Acta Dermosifiliogr 2008; 99: 199–206.
Dworkin AM, Ridd K, Bautista D, Allain DC, Iwenofu OH, Roy R et al. Germline variation controls the architecture of somatic alterations in tumors. PLoS Genet 2010; 6: e1001136.
Desmaze C, Soria JC, Freulet-Marriere MA, Mathieu N, Sabatier L . Telomere-driven genomic instability in cancer cells. Cancer Lett 2003; 194: 173–182.
Lansdorp PM . Telomeres and disease. EMBO J 2009; 28: 2532–2540.
Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J et al. Sunburn and p53 in the onset of skin cancer. Nature 1994; 372: 773–776.
Ermler S, Krunic D, Knoch TA, Moshir S, Mai S, Greulich-Bode KM et al. Cell cycle-dependent 3D distribution of telomeres and telomere repeat-binding factor 2 (TRF2) in HaCaT and HaCaT-myc cells. Eur J Cell Biol 2004; 83: 681–690.
Chuang TC, Moshir S, Garini Y, Chuang AY, Young IT, Vermolen B et al. The three-dimensional organization of telomeres in the nucleus of mammalian cells. BMC Biol 2004; 2: 12.
Louis SF, Vermolen BJ, Garini Y, Young IT, Guffei A, Lichtensztejn Z et al. c-Myc induces chromosomal rearrangements through telomere and chromosome remodeling in the interphase nucleus. Proc Natl Acad Sci USA 2005; 102: 9613–9618.
Murnane JP . Telomere dysfunction and chromosome instability. Mutat Res 2012; 730: 28–36.
Krunic D, Moshir S, Greulich-Bode KM, Figueroa R, Cerezo A, Stammer H et al. Tissue context-activated telomerase in human epidermis correlates with little age-dependent telomere loss. Biochim Biophys Acta 2009; 1792: 297–308.
Perrem K, Lynch A, Al NF, Leader M, Elaine K . The different telomere lengths in basal and squamous cell carcinomas also differ between the nontransplant and renal transplant population. Hum Pathol 2008; 39: 1034–1041.
Shay JW, Wright WE . Ageing and cancer: the telomere and telomerase connection. Novartis Found Symp 2001; 235: 116–125.
Bachor C, Bachor OA, Boukamp P . Telomerase is active in normal gastrointestinal mucosa and not up-regulated in precancerous lesions. J Cancer Res Clin Oncol 1999; 125: 453–460.
Perrem K, Lynch A, Conneely M, Wahlberg H, Murphy G, Leader M et al. The higher incidence of squamous cell carcinoma in renal transplant recipients is associated with increased telomere lengths. Hum Pathol 2007; 38: 351–358.
Fabricius EM, Kruse-Boitschenko U, Khoury R, Wildner GP, Raguse JD, Klein M et al. Localization of telomerase hTERT protein in frozen sections of basal cell carcinomas (BCC) and tumor margin tissues. Int J Oncol 2009; 35: 1377–1394.
Ren ZP, Ahmadian A, Ponten F, Nister M, Berg C, Lundeberg J et al. Benign clonal keratinocyte patches with p53 mutations show no genetic link to synchronous squamous cell precancer or cancer in human skin. Am J Pathol 1997; 150: 1791–1803.
Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science 1992; 258: 818–821.
Jones MJ, Jallepalli PV . Chromothripsis: chromosomes in crisis. Dev Cell 2012; 23: 908–917.
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE . Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988; 106: 761–771.
Lehman TA, Modali R, Boukamp P, Stanek J, Bennett WP, Welsh JA et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis 1993; 14: 833–839.
Cerezo A, Kalthoff H, Schuermann M, Schafer B, Boukamp P . Dual regulation of telomerase activity through c-Myc-dependent inhibition and alternative splicing of hTERT. J Cell Sci 2002; 115: 1305–1312.
Geigl JB, Uhrig S, Speicher MR . Multiplex-fluorescence in situ hybridization for chromosome karyotyping. Nat Protoc 2006; 1: 1172–1184.
Armanios M, Blackburn EH . The telomere syndromes. Nat Rev Genet 2012; 13: 693–704.
Xu L, Li S, Stohr BA . The role of telomere biology in cancer. Annu Rev Pathol 2012; 8: 49–78.
Zimmer C, Fabre E . Principles of chromosomal organization: lessons from yeast. J Cell Biol 2011; 192: 723–733.
Raz V, Vermolen BJ, Garini Y, Onderwater JJ, Mommaas-Kienhuis MA, Koster AJ et al. The nuclear lamina promotes telomere aggregation and centromere peripheral localization during senescence of human mesenchymal stem cells. J Cell Sci 2008; 121: 4018–4028.
Balasubramanian S, Hurley LH, Neidle S . Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat Rev Drug Discov 2011; 10: 261–275.
du Manoir S, Speicher MR, Joos S, Schrock E, Popp S, Dohner H et al. Detection of complete and partial chromosome gains and losses by comparative genomic in situ hybridization. Hum Genet 1993; 90: 590–610.
Boukamp P, Popp S, Altmeyer S, Hulsen A, Fasching C, Cremer T et al. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer 1997; 19: 201–214.
Harle-Bachor C, Boukamp P . Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci USA 1996; 93: 6476–6481.
Acknowledgements
We thank Angelika Lampe for her help in preparing the manuscript, Manuel Berning for constructive discussions and Iris Martin and Katrin Schmidt for their excellent technical assistance. We also thank Catherine Harwood, Charlotte Proby and Irene Leigh from the Cancer Research UK Skin Tumour Laboratory for providing us with the tumor material from the RT recipients. We further thank Dako (Denmark) for generously providing us with telomeric PNA probe. This work was supported by grants from the Deutsche Krebshilfe eV (Tumorstammzellverbund) and Bmbf (UVA Kompetenzverbund) (to PB), Tumorzentrum Heidelberg/Mannheim (to AJ and PB), the Deutsche Forschungsgemeinschaft (SFB873 to PB and SFB 829, Z2 to CM) and supported by contract research ‘Adulte epidermale Stammzellen’ of the Baden-Württemberg Stiftung’ (P-BWS-ASII/35).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Leufke, C., Leykauf, J., Krunic, D. et al. The telomere profile distinguishes two classes of genetically distinct cutaneous squamous cell carcinomas. Oncogene 33, 3506–3518 (2014). https://doi.org/10.1038/onc.2013.323
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.323