Abstract
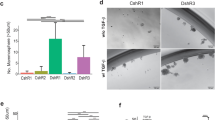
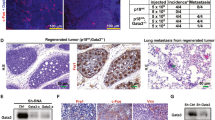
The key molecular events required for the formation of ductal carcinoma in situ (DCIS) and its progression to invasive breast carcinoma have not been defined. Here, we show that the nuclear receptor coactivator amplified in breast cancer 1 (AIB1) is expressed at low levels in normal breast but is highly expressed in DCIS lesions. This is of significance since reduction of AIB1 in human MCFDCIS cells restored a more normal three-dimensional mammary acinar structure. Reduction of AIB1 in MCFDCIS cells, both before DCIS development or in existing MCFDCIS lesions in vivo, inhibited tumor growth and led to smaller, necrotic lesions. AIB1 reduction in MCFDCIS cells was correlated with significant reduction in the CD24−/CD44+ breast cancer-initiating cell (BCIC) population, and a decrease in myoepithelial progenitor cells in the DCIS lesions in vitro and in vivo. The loss of AIB1 in MCFDCIS cells was also accompanied by a loss of expression of NOTCH 2, 3 and 4, JAG2, HES1, GATA3, human epidermal growth factor receptor 2 (HER2) and HER3 in vivo. These signaling molecules have been associated with differentiation of breast epithelial progenitor cells. These data indicate that AIB1 has a central role in the initiation and maintenance of DCIS and that reduction of AIB1 causes loss of BCIC, loss of components of the NOTCH, HER2 and HER3 signaling pathways and fewer DCIS myoepithelial progenitor cells in vivo. We propose that increased expression of AIB1, through the maintenance of BCIC, facilitates formation of DCIS, a necessary step before development of invasive disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Radford DM, Phillips NJ, Fair KL, Ritter JH, Holt M, Donis-Keller H . Allelic loss and the progression of breast cancer. Cancer Res 1995; 55: 5180–5183.
Stratton MR, Collins N, Lakhani SR, Sloane JP . Loss of heterozygosity in ductal carcinoma in situ of the breast. J Pathol 1995; 175: 195–201.
Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG et al. In situ analyses of genome instability in breast cancer. Nat Genet 2004; 36: 984–988.
Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA 2003; 100: 5974–5979.
Porter D, Lahti-Domenici J, Keshaviah A, Bae YK, Argani P, Marks J et al. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res 2003; 1: 362–375.
Espina V, Liotta LA . What is the malignant nature of human ductal carcinoma in situ? Nat Rev 2011; 11: 68–75.
Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 1997; 277: 965–968.
York B, O’Malley BW . Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem 2010; 285: 38743–38750.
Lahusen T, Henke RT, Kagan BL, Wellstein A, Riegel AT . The role and regulation of the nuclear receptor co-activator AIB1 in breast cancer. Breast Cancer Res Treat 2009; 116: 225–237.
Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 2004; 6: 263–274.
Kuang SQ, Liao L, Wang S, Medina D, O'Malley BW, Xu J . Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res 2005; 65: 7993–8002.
Fereshteh MP, Tilli MT, Kim SE, Xu J, O'Malley BW, Wellstein A et al. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res 2008; 68: 3697–3706.
Nakles RE, Shiffert MT, Díaz-Cruz ES, Cabrera MC, Alotaiby M, Miermont AM et al. Altered AIB1 or AIB1Δ3 expression impacts ERα effects on mammary gland stromal and epithelial content. Mol Endocrinol 2011; 25: 549–563.
Miller FR, Santner SJ, Tait L, Dawson PJ . MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 2000; 92: 1185–1186.
Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 2008; 13: 394–406.
Chien CD, Kirilyuk A, Li JV, Zhang W, Lahusen T, Schmidt MO et al. Role of the nuclear receptor coactivator AIB1-{Delta}4 in the control of gene transcription. J Biol Chem 2011; 286: 26813–26827.
Qutob MS, Bhattacharjee RN, Pollari E, Yee SP, Torchia J . Microtubule-dependent subcellular redistribution of the transcriptional coactivator p/CIP. Mol Cell Biol 2002; 22: 6611–6626.
Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R et al. SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol Cell 2010; 37: 321–332.
Miller FR . Xenograft models of premalignant breast disease. J Mammary Gland Biol Neopl 2000; 5: 379–391.
Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS . The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 2002; 111: 29–40.
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS . ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 2001; 3: 785–792.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Korkaya H, Wicha MS . Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs 2007; 21: 299–310.
Stingl J, Raouf A, Emerman JT, Eaves CJ . Epithelial progenitors in the normal human mammary gland. J Mamm Gland Biol Neopl 2005; 10: 49–59.
Xu J, Wu RC, O'Malley BW . Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev 2009; 9: 615–630.
Cody HS . Sentinel lymph node biopsy for DCIS: are we approaching consensus? Ann Surg Oncol 2007; 14: 2179–2181.
Sung YM, Xu X, Sun J, Mueller D, Sentissi K, Johnson P et al. Tumor suppressor function of Syk in human MCF10A in vitro and normal mouse mammary epithelium in vivo. PLoS One 2009; 4: e7445.
Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 2008; 3: 429–441.
Korkaya H, Wicha MS . HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res 2009; 15: 1845–1847.
Pradeep CR, Kostler WJ, Lauriola M, Granit RZ, Zhang F, Jacob-Hirsch J et al. Modeling ductal carcinoma in situ: a HER2-Notch3 collaboration enables luminal filling. Oncogene 2012; 31: 907–917.
Nofech-Mozes S, Spayne J, Rakovitch E, Hanna W . Prognostic and predictive molecular markers in DCIS: a review. Adv Anat Pathol 2005; 12: 256–264.
Korkaya H, Paulson A, Iovino F, Wicha MS . HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008; 27: 6120–6130.
Balko JM, Miller TW, Morrison MM, Hutchinson K, Young C, Rinehart C et al. The receptor tyrosine kinase ErbB3 maintains the balance between luminal and basal breast epithelium. Proc Natl Acad Sci USA 2012; 109: 221–226.
Lauritsen KJ, List HJ, Reiter R, Wellstein A, Riegel AT . A role for TGF-beta in estrogen and retinoid mediated regulation of the nuclear receptor coactivator AIB1 in MCF-7 breast cancer cells. Oncogene 2002; 21: 7147–7155.
Torres-Arzayus MI, Zhao J, Bronson R, Brown M . Estrogen-dependent and estrogen-independent mechanisms contribute to AIB1-mediated tumor formation. Cancer Res 2010; 70: 4102–4111.
Debnath J, Muthuswamy SK, Brugge JS . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30: 256–268.
Lee GY, Kenny PA, Lee EH, Bissell MJ . Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 2007; 4: 359–365.
Oh A, List H-J, Reiter R, Mani A, Zhang Y, Gehan E et al. The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res 2004; 64: 8299–8308.
Cavalli LR, Man YG, Schwartz AM, Rone JD, Zhang Y, Urban CA et al. Amplification of the BP1 homeobox gene in breast cancer. Cancer Genet Cytogenet 2008; 187: 19–24.
Cavalli LR, Santos SC, Broustas CG, Rone JD, Kasid UN, Haddad BR . Assignment of the BLID gene to 11q24.1 by fluorescence in situ hybridization. Cancer Genet Cytogenet 2008; 186: 120–121.
Al-Otaiby M, Tassi E, Schmidt MO, Chien CD, Baker T, Salas AG et al. Role of the nuclear receptor coactivator AIB1/SRC-3 in angiogenesis and wound healing. Am J Pathol 2012; 180: 1474–1484.
Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 2006; 124: 1283–1298.
Rosenfield SM, Bowden ET, Cohen-Missner S, Gibby KA, Ory V, Henke RT et al. Pleiotrophin (PTN) expression and function and in the mouse mammary gland and mammary epithelial cells. PLoS One 2012; 7: e47876.
Schmittgen TD, Livak KJ . Analyzing real-time PCR data by the comparative C(T) method. Nat Protocols 2008; 3: 1101–1108.
Acknowledgements
This work was funded by the Department of Defense Breast Cancer Research Program BC098103 (VO) and by NIH/NCI Grants CA113477 (ATR). This project was also supported by Award Number P30CA051008 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ory, V., Tassi, E., Cavalli, L. et al. The nuclear coactivator amplified in breast cancer 1 maintains tumor-initiating cells during development of ductal carcinoma in situ. Oncogene 33, 3033–3042 (2014). https://doi.org/10.1038/onc.2013.263
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.263
Keywords
This article is cited by
-
The PPARγ agonist efatutazone delays invasive progression and induces differentiation of ductal carcinoma in situ
Breast Cancer Research and Treatment (2018)