Abstract
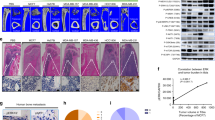
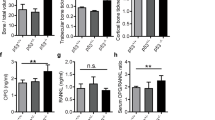
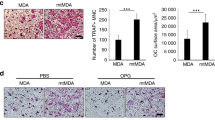
The skeleton is the most common metastatic site for breast cancer, with bone metastasis causing pain as well as risk of pathological fractures. Interaction between tumors and the bone microenvironment creates a vicious cycle that accelerates both bone destruction and cancer progression. This study is the first to analyze the soluble factors secreted by breast tumor-associated osteoblasts (TAOBs), which are responsible for promoting cancer progression. The addition of CXCL5 (chemokine (C-X-C motif) ligand 5), present in large amounts in TAOB-condition medium (TAOB-CM), mimicked the inductive effect of TAOB-CM on breast cancer epithelial–mesenchymal transition, migration and invasion. In contrast, inhibition of CXCL5 in OBs decreased TAOB-mediated cancer progression. Inducement of MCF-7 and MDA-MB-231 cancer progression by TAOB-derived CXCL5 is associated with increased Raf/MEK/ERK activation, and mitogen- and stress-activated protein kinase 1 (MSK1) and Elk-1 phosphorylation, as well as Snail upregulation. Activation of Elk-1 facilitates recruitment of phosphorylated MSK1, which in turn enhances histone H3 acetylation and phosphorylation (serine 10) of Snail promoter, resulting in Snail enhancement and E-cadherin downregulation. Moreover, mice treated with anti-CXCL5 antibodies showed decreased metastasis of 4T1 breast cancer cells. Our study suggests that inhibition of CXCL5-mediated ERK/Snail signaling is an attractive therapeutic target for treating metastases in breast cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Onishi T, Hayashi N, Theriault RL, Hortobagyi GN, Ueno NT . Future directions of bone-targeted therapy for metastatic breast cancer. Nat Rev Clin Oncol 2010; 7: 641–651.
Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev 2010; 36: 615–620.
Chen Y, Shi HY, Stock SR, Stern PH, Zhang M . Regulation of breast cancer-induced bone lesions by β-catenin protein signaling. J Biol Chem 2011; 49: 42 575–42584.
Van't Veer LJ, Weigelt B . Road map to metastasis. Nat Med 2003; 9: 999–1000.
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Yuen HF, Chan YK, Grills C, McCrudden CM, Gunasekharan V, Shi Z et al. Polyomavirus enhancer activator 3 protein promotes breast cancer metastatic progression through Snail-induced epithelial-mesenchymal transition. J Pathol 2011; 224: 78–89.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Yang J, Weinberg RA . Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–829.
Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA 2010; 107: 15 449–15454.
Jordà M, Vinyals A, Marazuela A, Cubillo E, Olmeda D, Valero E et al. Id-1 is induced in MDCK epithelial cells by activated Erk/MAPK pathway in response to expression of the Snail and E47 transcription factors. Exp Cell Res 2007; 313: 2389–2403.
Dunn KL, Espino PS, Drobic B, He S, Davie JR . The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem. Cell Biol 2005; 83: 1–14.
Espino PS, Li L, He S, Yu J, Davie JR . Chromatin modification of the trefoil factor 1 gene in human breast cancer cells by the Ras/mitogen-activated protein kinase pathway. Cancer Res 2006; 66: 4610–4616.
Pacheco-Rodriguez G, Kumaki F, Steagall WK, Zhang Y, Ikeda Y, Lin JP et al. Chemokine-enhanced chemotaxis of lymphangioleiomyomatosis cells with mutations in the tumor suppressor TSC2 gene. J Immunol 2009; 182: 1270–1277.
Bièche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L et al. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer 2007; 14: 1039–1052.
Kuo PL, Hung JY, Huang SK, Chou SH, Cheng DE, Jong YJ et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol 2011; 186: 1521–1530.
Georges S, Ruiz Velasco C, Trichet V, Fortun Y, Heymann D, Padrines M . Proteases and bone remodelling. Cytokine Growth Factor Rev 2009; 20: 29–41.
Hsu YL, Huang MS, Yang CJ, Hung JY, Wu LY, Kuo PL . Lung tumor-associated osteoblast-derived bone morphogenetic protein-2 increased epithelial-to-mesenchymal transition of cancer by Runx2/Snail signaling pathway. J Biol Chem 2011; 286: 37 335–37346.
Chirgwin JM, Guise TA . Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr 2000; 10: 159–178.
Kuo PL, Chen YH, Chen TC, Shen KH, Hsu YL . CXCL5/ENA78 increased cell migration and epithelial-to-mesenchymal transition of hormone-independent prostate cancer by early growth response-1/snail signaling pathway. J Cell Physiol 2011; 226: 1224–1231.
Besnard A, Bouveyron N, Kappes V, Pascoli V, Pagès C, Heck N et al. Alterations of molecular and behavioral responses to cocaine by selective inhibition of Elk-1 phosphorylation. J Neurosci 2011; 31: 14296–14307.
Roodman GD . Mechanisms of bone metastasis. N Engl J Med 2004; 350: 1655–1664.
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Christiansen JJ, Rajasekaran AK . Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 2006; 66: 8319–8326.
Thiery JP . Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003; 15: 740–746.
Nieto MA . The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 2011; 27: 347–376.
Peinado H, Olmeda D, Cano A . Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–428.
Olmeda D, Jordá M, Peinado H, Fabra A, Cano A . Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 2007; 26: 1862–1874.
Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 2002; 21: 3241–3246.
Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT, Wu CW et al. Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 2011; 20: 3814–3823.
Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 2005; 8: 197–209.
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000; 2: 76–83.
Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan ZK et al. Extracellular signal-regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression. Cancer Res 2009; 69: 9228–9235.
Mueller H, Flury N, Eppenberger-Castori S, Kueng W, David F, Eppenberger U . Potential prognostic value of mitogen-activated protein kinase activity for disease-free survival of primary breast cancer patients. Int J Cancer 2000; 89: 384–388.
Sivaraman VS, Wang H, Nuovo GJ, Malbon CC . Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest 1997; 99: 1478–1483.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007; 1773: 1263–1284.
Zhang HM, Li L, Papadopoulou N, Hodgson G, Evans E, Galbraith M et al. Mitogen-induced recruitment of ERK and MSK to SRE promoter complexes by ternary complex factor Elk-1. Nucleic Acids Res 2008; 36: 2594–2607.
Cassinat B, Zassadowski F, Ferry C, Llopis L, Bruck N, Lainey E et al. New role for granulocyte colony-stimulating factor-induced extracellular signal-regulated kinase 1/2 in histone modification and retinoic acid receptor α recruitment to gene promoters: relevance to acute promyelocytic leukemia cell differentiation. Mol Cell Biol 2011; 31: 1409–1418.
Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I et al. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell 2006; 24: 367–381.
Acknowledgements
This study is supported by grants from the National Science Council of Taiwan (NSC 99-2320-B-037-017-MY3), the Excellence for Cancer Research Center Grant, the Department of Health, Executive Yuan, Taipei, Taiwan (DOH101-TD-C-111-002), the Kaohsiung Medical University Hospital (KMUH 99–9I04) and the Kaohsiung Medical University Research Foundation (KMUER008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hsu, YL., Hou, MF., Kuo, PL. et al. Breast tumor-associated osteoblast-derived CXCL5 increases cancer progression by ERK/MSK1/Elk-1/Snail signaling pathway. Oncogene 32, 4436–4447 (2013). https://doi.org/10.1038/onc.2012.444
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.444
Keywords
This article is cited by
-
Targeting CXCL5 in Pancreatic Cancer Cells Inhibits Cancer Xenograft Growth by Reducing Proliferation and Inhibiting EMT Progression
Digestive Diseases and Sciences (2023)
-
ADSCs stimulated by resistin promote breast cancer cell malignancy via CXCL5 in a breast cancer coculture model
Scientific Reports (2022)
-
Breast cancer immune microenvironment: from pre-clinical models to clinical therapies
Breast Cancer Research and Treatment (2022)
-
Overexpression of Lrp5 enhanced the anti-breast cancer effects of osteocytes in bone
Bone Research (2021)
-
EGR1 and RXRA transcription factors link TGF-β pathway and CCL2 expression in triple negative breast cancer cells
Scientific Reports (2021)