Abstract
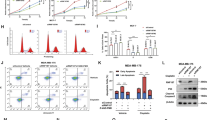
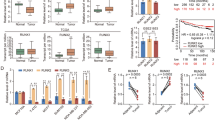
Emerging evidence demonstrates that RUNX3 is a tumor suppressor in breast cancer. Inactivation of RUNX3 in mice results in spontaneous mammary gland tumors, and decreased or silenced expression of RUNX3 is frequently found in breast cancer cell lines and human breast cancer samples. However, the underlying mechanism for initiating RUNX3 inactivation in breast cancer remains elusive. Here, we identify prolyl isomerase Pin1, which is often overexpressed in breast cancer, as a key regulator of RUNX3 inactivation. In human breast cancer cell lines and breast cancer samples, expression of Pin1 inversely correlates with the expression of RUNX3. In addition, Pin1 recognizes four phosphorylated Ser/Thr-Pro motifs in RUNX3 via its WW domain. Binding of Pin1 to RUNX3 suppresses the transcriptional activity of RUNX3. Furthermore, Pin1 reduces the cellular levels of RUNX3 in an isomerase activity-dependent manner by inducing the ubiquitination and proteasomal degradation of RUNX3. Knocking down Pin1 enhances the cellular levels and transcriptional activity of RUNX3 by inhibiting the ubiquitination and degradation of RUNX3. Our results identify Pin1 as a new regulator of RUNX3 inactivation in breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Katzenellenbogen BS, Katzenellenbogen JA . Estrogen receptor transcription and transactivation: Estrogen receptor alpha and estrogen receptor beta: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res 2000; 2: 335–344.
Cheskis BJ, Greger JG, Nagpal S, Freedman LP . Signaling by estrogens. J Cell Physiol 2007; 213: 610–617.
Lee EY, Muller WJ . Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol 2010; 2: a003236.
Huang B, Qu Z, Ong CW, Tsang YH, Xiao G, Shapiro D et al. RUNX3 acts as a tumor suppressor in breast cancer by targeting estrogen receptor alpha. Oncogene 2011; 31: 527–534.
Ito Y . Oncogenic potential of the RUNX gene family: ‘overview’. Oncogene 2004; 23: 4198–4208.
Subramaniam MM, Chan JY, Soong R, Ito K, Ito Y, Yeoh KG et al. RUNX3 inactivation by frequent promoter hypermethylation and protein mislocalization constitute an early event in breast cancer progression. Breast Cancer Res Treat 2009; 113: 113–121.
Jiang Y, Tong D, Lou G, Zhang Y, Geng J . Expression of RUNX3 gene, methylation status and clinicopathological significance in breast cancer and breast cancer cell lines. Pathobiology 2008; 75: 244–251.
Lau QC, Raja E, Salto-Tellez M, Liu Q, Ito K, Inoue M et al. RUNX3 is frequently inactivated by dual mechanisms of protein mislocalization and promoter hypermethylation in breast cancer. Cancer Res 2006; 66: 6512–6520.
Subramaniam MM, Chan JY, Yeoh KG, Quek T, Ito K, Salto-Tellez M . Molecular pathology of RUNX3 in human carcinogenesis. Biochim Biophys Acta 2009; 1796: 315–331.
Chen LF . Tumor suppressor function of RUNX3 in breast cancer. J Cell Biochem 2012; 113: 1470–1477.
Chuang LS, Ito Y . RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene 2010; 29: 2605–2615.
Hwang KT, Han W, Bae JY, Hwang SE, Shin HJ, Lee JE et al. Downregulation of the RUNX3 gene by promoter hypermethylation and hemizygous deletion in breast cancer. J Korean Med Sci 2007; 22 (Suppl): S24–S31.
Bae SC, Lee YH . Phosphorylation, acetylation and ubiquitination: the molecular basis of RUNX regulation. Gene 2006; 366: 58–66.
Bae JS, Jang MK, Hong S, An WG, Choi YH, Kim HD et al. Phosphorylation of NF-kappa B by calmodulin-dependent kinase IV activates anti-apoptotic gene expression. Biochem Biophys Res Commun 2003; 305: 1094–1098.
Lu KP, Zhou XZ . The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol 2007; 8: 904–916.
Liou YC, Zhou XZ, Lu KP . Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci 2011; 36: 501–514.
Wulf G, Ryo A, Liou YC, Lu KP . The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res 2003; 5: 76–82.
Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J 2001; 20: 3459–3472.
Liou YC, Ryo A, Huang HK, Lu PJ, Bronson R, Fujimori F et al. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci USA 2002; 99: 1335–1340.
Rajbhandari P, Finn G, Solodin NM, Singarapu KK, Sahu SC, Markley JL et al. Regulation of ERalpha N-terminus conformation and function by peptidyl prolyl isomerase Pin1. Mol Cell Biol 2011; 32: 445–457.
Yi P, Wu RC, Sandquist J, Wong J, Tsai SY, Tsai MJ et al. Peptidyl-prolyl isomerase 1 (Pin1) serves as a coactivator of steroid receptor by regulating the activity of phosphorylated steroid receptor coactivator 3 (SRC-3/AIB1). Mol Cell Biol 2005; 25: 9687–9699.
Rustighi A, Tiberi L, Soldano A, Napoli M, Nuciforo P, Rosato A et al. The prolyl-isomerase Pin1 is a Notch1 target that enhances Notch1 activation in cancer. Nat Cell Biol 2009; 11: 133–142.
Reineke EL, Lam M, Liu Q, Liu Y, Stanya KJ, Chang KS et al. Degradation of the tumor suppressor PML by Pin1 contributes to the cancer phenotype of breast cancer MDA-MB-231 cells. Mol Cell Biol 2008; 28: 997–1006.
Lim JH, Liu Y, Reineke E, Kao HY . The MAPK ERK2 phosphorylates and promotes Pin1-dependent promyelocytic leukemia protein (PML) turnover. J Biol Chem 2011; 286: 44403–44411.
Davis FM, Tsao TY, Fowler SK, Rao PN . Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA 1983; 80: 2926–2930.
Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 1997; 278: 1957–1960.
Lu PJ, Zhou XZ, Liou YC, Noel JP, Lu KP . Critical role of WW domain phosphorylation in regulating phosphoserine binding activity and Pin1 function. J Biol Chem 2002; 277: 2381–2384.
Hanai J, Chen LF, Kanno T, Ohtani-Fujita N, Kim WY, Guo WH et al. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Calpha promoter. J Biol Chem 1999; 274: 31577–31582.
Schutkowski M, Bernhardt A, Zhou XZ, Shen M, Reimer U, Rahfeld JU et al. Role of phosphorylation in determining the backbone dynamics of the serine/threonine-proline motif and Pin1 substrate recognition. Biochemistry 1998; 37: 5566–5575.
Zhang M, Xie R, Hou W, Wang B, Shen R, Wang X et al. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci 2009; 122: 1382–1389.
Butt AJ, Caldon CE, McNeil CM, Swarbrick A, Musgrove EA, Sutherland RL . Cell cycle machinery: links with genesis and treatment of breast cancer. Adv Exp Med Biol 2008; 630: 189–205.
Jin YH, Jeon EJ, Li QL, Lee YH, Choi JK, Kim WJ et al. Transforming growth factor-beta stimulates p300-dependent RUNX3 acetylation, which inhibits ubiquitination-mediated degradation. J Biol Chem 2004; 279: 29409–29417.
Nakano A, Koinuma D, Miyazawa K, Uchida T, Saitoh M, Kawabata M et al. Pin1 down-regulates transforming growth factor-beta (TGF-beta) signaling by inducing degradation of Smad proteins. J Biol Chem 2009; 284: 6109–6115.
Huang G, Shigesada K, Ito K, Wee HJ, Yokomizo T, Ito Y . Dimerization with PEBP2beta protects RUNX1/AML1 from ubiquitin-proteasome-mediated degradation. EMBO J 2001; 20: 723–733.
Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 2000; 6: 873–883.
Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG . Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol 2004; 164: 1727–1737.
Wulf G, Finn G, Suizu F, Lu KP . Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol 2005; 7: 435–441.
Tsang YH, Lamb A, Romero-Gallo J, Huang B, Ito K, Peek RM et al. Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for proteasome-mediated degradation. Oncogene 2010; 29: 5643–5650.
Zhang DH, Salto-Tellez M, Chiu LL, Shen L, Koay ES . Tissue microarray study for classification of breast tumors. Life Sci 2003; 73: 3189–3199.
Chen LF, Mu Y, Greene WC . Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J 2002; 21: 6539–6548.
Acknowledgements
We thank Drs Lu KP and Kao HY for the gift of reagents and members in the Chen lab for discussion. This work is supported in part by fund provided by UIUC (to LFC) and NIH grants DK-085158 (to LFC). YHT is an A*STAR-Illinois Partnership fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Nicole Tsang, YH., Wu, XW., Lim, JS. et al. Prolyl isomerase Pin1 downregulates tumor suppressor RUNX3 in breast cancer. Oncogene 32, 1488–1496 (2013). https://doi.org/10.1038/onc.2012.178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.178
Keywords
This article is cited by
-
RUNX3 inactivates oncogenic MYC through disruption of MYC/MAX complex and subsequent recruitment of GSK3β-FBXW7 cascade
Communications Biology (2023)
-
Prolyl isomerase PIN1 regulates the stability, transcriptional activity and oncogenic potential of BRD4
Oncogene (2017)
-
The isomerase PIN1 controls numerous cancer-driving pathways and is a unique drug target
Nature Reviews Cancer (2016)
-
Threonine 209 phosphorylation on RUNX3 by Pak1 is a molecular switch for its dualistic functions
Oncogene (2016)
-
The role of Pin1 in the development and treatment of cancer
Archives of Pharmacal Research (2016)