Abstract
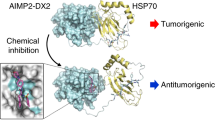
Human heat shock protein 27 (Hsp27, HspB1) is an anti-apoptotic protein characterized for its tumorigenic and metastatic properties, and now referenced as a major therapeutic target in many types of cancer. Hsp27 biochemical properties rely on a structural oligomeric and dynamic organization. Downregulation by small interfering RNA or inhibition with dominant-negative mutant have proven their efficiency to counteract the anti-apoptotic and protective properties of Hsp27. In this study, we report the isolation and characterization of Hsp27-targeted molecules interfering with its structural organization. Using the peptide aptamer (PA) strategy, we isolated PAs that specifically interact with Hsp27 and not with the other members of the small heat shock protein family. In mammalian cell cultures, PAs expression perturbed the dimerization and oligomerization of Hsp27, and acted as negative regulators of the anti-apoptotic and cytoprotective activities of this protein. Further studies analyzing SQ20B cell xenografts in immunocompromised mice showed that PAs strongly reduced tumor development through cell cycle arrest. Our data suggest that PAs could provide a potential tool to develop strategies for the discovery of Hsp27 chemical inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R et al. (2008). Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys 70: 543–553.
Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A et al. (2007). Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett 581: 3665–3674.
Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C . (2005). Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal 7: 414–422.
Baines IC, Colas P . (2006). Peptide aptamers as guides for small-molecule drug discovery. Drug Discov Today 11: 334–341.
Bickle MB, Dusserre E, Moncorge O, Bottin H, Colas P . (2006). Selection and characterization of large collections of peptide aptamers through optimized yeast two-hybrid procedures. Nat Protoc 1: 1066–1091.
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C et al. (2000a). Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2: 645–652.
Bruey JM, Paul C, Fromentin A, Hilpert S, Arrigo AP, Solary E et al. (2000b). Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene 19: 4855–4863.
Buerger C, Nagel-Wolfrum K, Kunz C, Wittig I, Butz K, Hoppe-Seyler F et al. (2003). Sequence-specific peptide aptamers, interacting with the intracellular domain of the epidermal growth factor receptor, interfere with Stat3 activation and inhibit the growth of tumor cells. J Biol Chem 278: 37610–37621.
Cervantes-Gomez F, Nimmanapalli R, Gandhi V . (2009). Transcription inhibition of heat shock proteins: a strategy for combination of 17-allylamino-17-demethoxygeldanamycin and actinomycin d. Cancer Res 69: 3947–3954.
Charette SJ, Landry J . (2000). The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann N Y Acad Sci 926: 126–131.
Chattopadhyay A, Tate SA, Beswick RW, Wagner SD, Ko Ferrigno P . (2006). A peptide aptamer to antagonize BCL-6 function. Oncogene 25: 2223–2233.
Ciocca DR, Calderwood SK . (2005). Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10: 86–103.
Diaz-Latoud C, Buache E, Javouhey E, Arrigo AP . (2005). Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal 7: 436–445.
Estojak J, Brent R, Golemis EA . (1995). Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol 15: 5820–5829.
Finley Jr RL, Brent R . (1994). Interaction mating reveals binary and ternary connections between Drosophila cell cycle regulators. Proc Natl Acad Sci USA 91: 12980–12984.
Gonin S, Diaz-Latoud C, Richard MJ, Ursini MV, Imbo A, Manero F et al. (1999). p53/T-antigen complex disruption in T-antigen transformed NIH3T3 fibroblasts exposed to oxidative stress: correlation with the appearance of a Fas/APO-1/CD95 dependent, caspase independent, necrotic pathway. Oncogene 18: 8011–8023.
Gyuris J, Golemis E, Chertkov H, Brent R . (1993). Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell 75: 791–803.
Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R et al. (2009). Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther 17: 1387–1394.
Hansen RK, Parra I, Lemieux P, Oesterreich S, Hilsenbeck SG, Fuqua SA . (1999). Hsp27 overexpression inhibits doxorubicin-induced apoptosis in human breast cancer cells. Breast Cancer Res Treat 56: 187–196.
Javouhey E, Gibert B, Arrigo AP, Diaz JJ, Diaz-Latoud C . (2008). Protection against heat and staurosporine mediated apoptosis by the HSV-1 US11 protein. Virology 376: 31–41.
Kim EH, Lee HJ, Lee DH, Bae S, Soh JW, Jeoung D et al. (2007). Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. Cancer Res 67: 6333–6341.
Laemmli UK . (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA et al. (1992). Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem 267: 794–803.
Matsui Y, Hadaschik BA, Fazli L, Andersen RJ, Gleave ME, So AI . (2009). Intravesical combination treatment with antisense oligonucleotides targeting heat shock protein-27 and HTI-286 as a novel strategy for high-grade bladder cancer. Mol Cancer Ther 8: 2402–2411.
Mehlen P, Briolay J, Smith L, Diaz-latoud C, Fabre N, Pauli D et al. (1993). Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type or deletion mutants of the Drosophila 27-kDa heat-shock protein. Eur J Biochem 215: 277–284.
Mehlen P, Hickey E, Weber LA, Arrigo AP . (1997). Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFalpha in NIH-3T3-ras cells. Biochem Biophys Res Commun 241: 187–192.
Mehlen P, Schulze-Osthoff K, Arrigo AP . (1996). Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem 271: 16510–16514.
Nouvion AL, Thibaut J, Lohez OD, Venet S, Colas P, Gillet G et al. (2007). Modulation of Nr-13 antideath activity by peptide aptamers. Oncogene 26: 701–710.
O'Callaghan-Sunol C, Gabai VL, Sherman MY . (2007). Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res 67: 11779–11788.
Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V et al. (2000). Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J 19: 4310–4322.
Parcellier A, Brunet M, Schmitt E, Col E, Didelot C, Hammann A et al. (2006). HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. FASEB J 20: 1179–1181.
Paul C, Simon S, Gibert B, Virot S, Manero F, Arrigo AP . (2010). Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27). Exp Cell Res 316: 1535–1552.
Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T et al. (2003). Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278: 27828–27835.
Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A et al. (1998). A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20: 92–95.
Zhang Y, Shen X . (2007). Heat shock protein 27 protects L929 cells from cisplatin-induced apoptosis by enhancing Akt activation and abating suppression of thioredoxin reductase activity. Clin Cancer Res 13: 2855–2864.
Acknowledgements
We wish to thank D Guillet for excellent technical assistance and Marc Bickle for helpful discussions. This work was supported by a research grant from the Comité du Rhône of la Ligue Contre le Cancer and the Région Rhône-Alpes and Retina France for APA. GB was supported by a PhD training grant from the La Région Rhône-Alpes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Gibert, B., Hadchity, E., Czekalla, A. et al. Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene 30, 3672–3681 (2011). https://doi.org/10.1038/onc.2011.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.73
Keywords
This article is cited by
-
Blood plasma derived extracellular vesicles (BEVs): particle purification liquid chromatography (PPLC) and proteomic analysis reveals BEVs as a potential minimally invasive tool for predicting response to breast cancer treatment
Breast Cancer Research and Treatment (2022)
-
Heat-shock proteins: chaperoning DNA repair
Oncogene (2020)
-
Advances in HSP27 and HSP90-targeting strategies for glioblastoma
Journal of Neuro-Oncology (2016)
-
Characterization of antiproliferative potential and biological targets of a copper compound containing 4′-phenyl terpyridine
JBIC Journal of Biological Inorganic Chemistry (2015)