Abstract
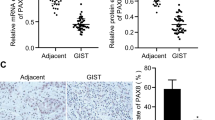
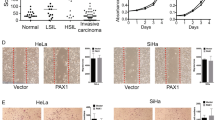
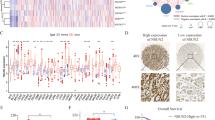
Using genome-wide methylation screening, we identified that paired box gene 5 (PAX5) is involved in human cancer development. However, the function of PAX5 in gastric cancer (GC) development is largely unclear. We analyzed its epigenetic inactivation, biological functions and clinical application in GC. PAX5 was silenced in seven out of eight GC cell lines. A significant downregulation was also detected in paired gastric tumors compared with adjacent non-cancerous tissues. The downregulation of PAX5 was closely linked to the promoter hypermethylation status and could be restored with demethylation treatment. Ectopic expression of PAX5 in silenced GC cell lines (AGS and BGC823) inhibited colony formation and cell viability, arrested cell cycle, induced apoptosis, suppressed cell migration and invasion and repressed tumorigenicity in nude mice. Consistent with the induction of apoptosis by PAX5 in vitro, terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL) staining showed significantly enhanced apoptotic cells in PAX5-expressed tumors compared with the vector control tumors. On the other hand, knockdown of PAX5 by PAX5–short hairpin RNA increased the cell viability and proliferation. The anti-tumorigenic function of PAX5 was revealed to be mediated by upregulating downstream targets of tumor protein 53 (p53), p21, BCL2-associated X protein, metastasis suppressor 1 and tissue inhibitors of metalloproteinase 1, and downregulating BCL2, cyclin D1, mesenchymal–epithelial transition factor (MET) and matrix metalloproteinase 1. Immunoprecipitation assay demonstrated that PAX5 directly bound to the promoters of p53 and MET. Moreover, PAX5 hypermethylation was detected in 77% (144 of 187) of primary GCs compared with 10.5% (2/19) of normal gastric tissues (P<0.0001). GC patients with PAX5 methylation had a significant poor survival compared with the unmethylated cases as demonstrated by Cox regression model and log-rank test. In conclusion, PAX5 is a novel functional tumor suppressor in gastric carcinogenesis. Detection of methylated PAX5 can be utilized as an independent prognostic factor in GC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- 5-Aza:
-
5-aza-2′-deoxycytidine
- BAX:
-
BCL2-associated X protein
- BGS:
-
bisulfite genomic sequencing
- ChIP:
-
chromatin immunoprecipitation
- CI:
-
confidence interval
- GC:
-
gastric cancer
- H. pylori :
-
Helicobacter pylori
- MET:
-
mesenchymal epithelial transition factor
- MMP1:
-
matrix metalloproteinase 1
- MSP:
-
methylation specific PCR
- MTSS1:
-
metastasis suppressor 1
- ORF:
-
open reading frame
- p53:
-
tumor protein 53
- PAX5 :
-
paired box gene 5
- PBS:
-
phosphate buffered saline
- PI:
-
propidium iodide
- qPCR:
-
quantitative polymerase chain reaction
- RR:
-
relative risk
- TIMP1:
-
tissue inhibitors of metalloproteinase 1
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labelling
References
Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, Maurer-Fogy I et al. (1992). Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev 6: 1589–1607.
Barberis A, Widenhorn K, Vitelli L, Busslinger M . (1990). A novel B-cell lineage-specific transcription factor present at early but not late stages of differentiation. Genes Dev 4: 849–859.
Baumann Kubetzko FB, Di Paolo C, Maag C, Meier R, Schafer BW, Betts DR et al. (2004). The PAX5 oncogene is expressed in N-type neuroblastoma cells and increases tumorigenicity of a S-type cell line. Carcinogenesis 25: 1839–1846.
Benvenuti S, Comoglio PM . (2007). The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol 213: 316–325.
Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF et al. (1991). Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251: 802–804.
Busslinger M, Klix N, Pfeffer P, Graninger PG, Kozmik Z . (1996). Deregulation of PAX-5 by translocation of the Emu enhancer of the IgH locus adjacent to two alternative PAX-5 promoters in a diffuse large-cell lymphoma. Proc Natl Acad Sci USA 93: 6129–6134.
Carotta S, Holmes ML, Pridans C, Nutt SL . (2006). Pax5 maintains cellular identity by repressing gene expression throughout B cell differentiation. Cell Cycle 5: 2452–2456.
Chambers AF, Matrisian LM . (1997). Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89: 1260–1270.
Cobaleda C, Schebesta A, Delogu A, Busslinger M . (2007). Pax5: the guardian of B cell identity and function. Nat Immunol 8: 463–470.
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825.
Giordano S, Zhen Z, Medico E, Gaudino G, Galimi F, Comoglio PM . (1993). Transfer of motogenic and invasive response to scatter factor/hepatocyte growth factor by transfection of human MET protooncogene. Proc Natl Acad Sci USA 90: 649–653.
Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM . (1994). Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res 54: 2095–2097.
He G, Siddik ZH, Huang Z, Wang R, Koomen J, Kobayashi R et al. (2005). Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 24: 2929–2943.
Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ . (1991). BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA 88: 6961–6965.
Holmes ML, Carotta S, Corcoran LM, Nutt SL . (2006). Repression of Flt3 by Pax5 is crucial for B-cell lineage commitment. Genes Dev 20: 933–938.
Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N et al. (1998). Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19: 187–191.
Kang GH, Lee HJ, Hwang KS, Lee S, Kim JH, Kim JS . (2003a). Aberrant CpG island hypermethylation of chronic gastritis, in relation to aging, gender, intestinal metaplasia, and chronic inflammation. Am J Pathol 163: 1551–1556.
Kang GH, Lee S, Kim JS, Jung HY . (2003b). Profile of aberrant CpG island methylation along multistep gastric carcinogenesis. Lab Invest 83: 519–526.
Kanteti R, Nallasura V, Loganathan S, Tretiakova M, Kroll T, Krishnaswamy S et al. (2009). PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription. Lab Invest 89: 301–314.
Kishimoto Y, Murakami Y, Shiraishi M, Hayashi K, Sekiya T . (1992). Aberrations of the p53 tumor suppressor gene in human non-small cell carcinomas of the lung. Cancer Res 52: 4799–4804.
Kovac CR, Emelyanov A, Singh M, Ashouian N, Birshtein BK . (2000). BSAP (Pax5)-importin alpha 1 (Rch1) interaction identifies a nuclear localization sequence. J Biol Chem 275: 16752–16757.
Kozmik Z, Wang S, Dorfler P, Adams B, Busslinger M . (1992). The promoter of the CD19 gene is a target for the B-cell-specific transcription factor BSAP. Mol Cell Biol 12: 2662–2672.
Lazzi S, Bellan C, Onnis A, De Falco G, Sayed S, Kostopoulos I et al. (2009). Rare lymphoid neoplasms coexpressing B- and T-cell antigens. The role of PAX-5 gene methylation in their pathogenesis. Hum Pathol 40: 1252–1261.
Lee BL, Kim WH, Jung J, Cho SJ, Park JW, Kim J et al. (2008). A hypoxia-independent up-regulation of hypoxia-inducible factor-1 by AKT contributes to angiogenesis in human gastric cancer. Carcinogenesis 29: 44–51.
Lee JH, Park SJ, Abraham SC, Seo JS, Nam JH, Choi C et al. (2004). Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene 23: 4646–4654.
Lee YG, Macoska JA, Korenchuk S, Pienta KJ . (2002). MIM, a potential metastasis suppressor gene in bladder cancer. Neoplasia 4: 291–294.
Levine AJ . (1989). The p53 tumor suppressor gene and gene product. Princess Takamatsu Symp 20: 221–230.
Liu W, Li X, Chu E, Go M, Xu L, Zhao G et al. (2011). Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology 53: 843–853.
Malkin D, Sexsmith E, Yeger H, Williams BR, Coppes MJ . (1994). Mutations of the p53 tumor suppressor gene occur infrequently in Wilms’ tumor. Cancer Res 54: 2077–2079.
Meza JL, Anderson J, Pappo AS, Meyer WH . (2006). Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children's Oncology Group. J Clin Oncol 24: 3844–3851.
Mhawech-Fauceglia P, Saxena R, Zhang S, Terracciano L, Sauter G, Chadhuri A et al. (2007). Pax-5 immunoexpression in various types of benign and malignant tumours: a high-throughput tissue microarray analysis. J Clin Pathol 60: 709–714.
Miyashita T, Reed JC . (1995). Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299.
Musgrove EA, Lee CS, Buckley MF, Sutherland RL . (1994). Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci USA 91: 8022–8026.
Nutt SL, Urbanek P, Rolink A, Busslinger M . (1997). Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev 11: 476–491.
Oltvai ZN, Milliman CL, Korsmeyer SJ . (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619.
Parr C, Jiang WG . (2009). Metastasis suppressor 1 (MTSS1) demonstrates prognostic value and anti-metastatic properties in breast cancer. Eur J Cancer 45: 1673–1683.
Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF et al. (1993). Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev 7: 1559–1571.
Rocha S, Martin AM, Meek DW, Perkins ND . (2003). p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol Cell Biol 23: 4713–4727.
Ruvolo PP, Deng X, May WS . (2001). Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 15: 515–522.
Sauter W, Rosenberger A, Beckmann L, Kropp S, Mittelstrass K, Timofeeva M et al. (2008). Matrix metalloproteinase 1 (MMP1) is associated with early-onset lung cancer. Cancer Epidemiol Biomarkers Prev 17: 1127–1135.
Souabni A, Cobaleda C, Schebesta M, Busslinger M . (2002). Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity 17: 781–793.
Stapleton P, Weith A, Urbanek P, Kozmik Z, Busslinger M . (1993). Chromosomal localization of seven PAX genes and cloning of a novel family member, PAX-9. Nat Genet 3: 292–298.
Stuart ET, Haffner R, Oren M, Gruss P . (1995). Loss of p53 function through PAX-mediated transcriptional repression. EMBO J 14: 5638–5645.
Tao Q, Huang H, Geiman TM, Lim CY, Fu L, Qiu GH et al. (2002). Defective de novo methylation of viral and cellular DNA sequences in ICF syndrome cells. Hum Mol Genet 11: 2091–2102.
Visse R, Nagase H . (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839.
Wang Y, Liu J, Smith E, Zhou K, Liao J, Yang GY et al. (2007). Downregulation of missing in metastasis gene (MIM) is associated with the progression of bladder transitional carcinomas. Cancer Invest 25: 79–86.
Ying J, Li H, Seng TJ, Langford C, Srivastava G, Tsao SW et al. (2006). Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene 25: 1070–1080.
Yu J, Chu ES, Wang R, Wang S, Wu CW, Wong VW et al. (2010). Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocytes and mice. Gastroenterology 138: 694–704.
Zwollo P, Desiderio S . (1994). Specific recognition of the blk promoter by the B-lymphoid transcription factor B-cell-specific activator protein. J Biol Chem 269: 15310–15317.
Acknowledgements
This study was supported by research grants of National Basic Research Program of China (973 Program, 2010CB529305), Hong Kong General Research Fund (473008), RFCID (11100022, 10090942), CUHK Group Research Scheme (3110043) and Scheme C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, X., Cheung, K., Ma, X. et al. Epigenetic inactivation of paired box gene 5, a novel tumor suppressor gene, through direct upregulation of p53 is associated with prognosis in gastric cancer patients. Oncogene 31, 3419–3430 (2012). https://doi.org/10.1038/onc.2011.511
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.511
Keywords
This article is cited by
-
Epigenetic activation of the elongator complex sensitizes gallbladder cancer to gemcitabine therapy
Journal of Experimental & Clinical Cancer Research (2021)
-
PAX5 activates telomerase activity and proliferation in keloid fibroblasts by transcriptional regulation of SND1, thus promoting keloid growth in burn-injured skin
Inflammation Research (2021)
-
PAX8 activates a p53-p21-dependent pro-proliferative effect in high grade serous ovarian carcinoma
Oncogene (2018)
-
Epigenetic silencing of GDF1 disrupts SMAD signaling to reinforce gastric cancer development
Oncogene (2016)
-
Ras association domain family member 10 suppresses gastric cancer growth by cooperating with GSTP1 to regulate JNK/c-Jun/AP-1 pathway
Oncogene (2016)