Abstract
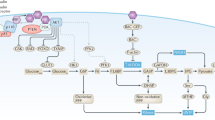
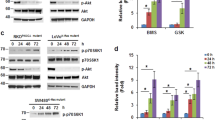
Women with type 2 diabetes mellitus (T2DM) are at a greater risk of developing and dying from breast cancer than women without T2DM. Insulin resistance and hyperinsulinemia underlie the pathogenesis of T2DM. In the MKR mouse model of insulin resistance, we have previously shown increased activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway in association with accelerated mammary tumor growth. In this study, we demonstrate that inhibiting PI3K with the oral pan-class I PI3K inhibitor, NVP-BKM120 reduced the growth of Met-1 and MCNeuA mammary tumor orthografts in the MKR mouse. NVP-BKM120 treatment decreased phosphorylation of Akt and S6 ribosomal protein (S6rp); no change in Erk1/2 phosphorylation was seen. Hyperglycemia, hypertriglyceridemia and greater hyperinsulinemia developed in the MKR mice treated with NVP-BKM120. We previously reported reduced tumor growth using intraperitoneal rapamycin in the MKR mouse, with the development of hyperglycemia and hypertriglyceridemia. Therefore, we examined whether the oral PI3K/mTOR inhibitor NVP-BEZ235 augmented the tumor suppressing effects of PI3K inhibition. We also investigated the effect of targeted PI3K/mTOR inhibition on PI3K/Akt/mTOR and Erk1/2 signaling, and the potential effects on glycemia. NVP-BEZ235 suppressed the growth of Met-1 and MCNeuA tumor orthografts, and decreased Akt and S6rp phosphorylation, despite increased Erk1/2 phosphorylation in Met-1 orthografts of MKR mice. Less marked hyperglycemia and hyperinsulinemia developed with NVP-BEZ235 than NVP-BKM120. Overall, the results of this study demonstrated that inhibiting PI3K/Akt/mTOR signaling with the oral agents NVP-BKM120 and NVP-BEZ235 decreased mammary tumor growth in the hyperinsulinemic MKR mouse. Inhibiting PI3K alone led to more severe metabolic derangement than inhibiting both PI3K and mTOR. Therefore, PI3K may be an important target for the treatment of breast cancer in women with insulin resistance. Monitoring for hyperglycemia and dyslipidemia should be considered when using these agents in humans, given the metabolic changes detected in this study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Agnoli C, Berrino F, Abagnato CA, Muti P, Panico S, Crosignani P et al. (2010). Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metab Cardiovasc Dis 20: 41–48.
Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweb S, Bharwani L et al. (2007). Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent-targeted therapy. JNCI 99: 694–705.
Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG et al. (2005). Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis 22: 47–59.
Campbell MJ, Wollish WS, Lobo M, Esserman LJ . (2002). Epithelial and fibroblast cell lines derived from a spontaneous mammary carcinoma in a MMTV/neu transgenic mouse. In vitro Cell Dev Biol Anim 38: 326–333.
Cannata D, Lann D, Wu Y, Elis S, Sun H, Yakar S et al. (2010). Elevated circulatory IGF-I promotes mammary gland development and proliferation. Endocrinology 151: 5751–5761.
Creighton CJ, Fu X, Hennessy B, Casa AJ, Zhang Y, Gonzalez-Angulo AM et al. (2010). Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res 12: R40.
Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL et al. (2001). Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 15: 1926–1934.
Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D . (2010). Mammalian target of rapamycin inhibition abrogates insulin-mediated mammary tumor progression in type 2 diabetes. Endocr Relat Cancer 17: 941–951.
Foukas LC, Berejeno IM, GrayA, Khwaja A, Vanhaesebroeck B . (2010). Activity of any class 1A PI3K isoform can sustain cell proliferation and survival. Proc Natl Acad Science 107: 11381–11386.
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y et al. (2002). Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20: 42–51.
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH et al. (2008). Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454: 776–779.
Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS et al. (2009). Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15: 429–440.
Kohn AD, Summers SA, Birnbaum MJ, Roth RA . (1996). Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 271: 31372–31378.
Liu LZ, Zhao HL, Zuo J, Ho SK, Chan JC, Meng Y et al. (2006). Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells. Mol Biol Cell 17: 2322–2330.
López-Knowles E, O'Toole SA, McNeil CM, Millar EK, Qiu MR, Crea P et al. (2010). PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer 126: 1121–1131.
Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y et al. (2010). Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res 70: 741–751.
O'Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J et al. (2010). Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther 9: 1489–1502.
Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L et al. (1990). Elevated insulin receptor content in human breast cancer. J Clin Invest 86: 1503–1510.
Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA et al. (2010). Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat 122: 859–865.
Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL et al. (2011). Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 29: 40–46.
Serra V, Markman B, Scaitriti M, Eichorn PJ, Valero V, Guzman M et al. (2008). NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 68: 8022–8030.
Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S et al. (2011). PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 30: 2547–2557.
Sopasakis VR, Liu P, Suzuki R, Kondo T, Winnay J, Tran TT et al. (2010). Specific roles of the p110alpha isoform of phosphatidylinositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab 11: 220–230.
Tan SX, Ng Y, James DE . (2010). Akt inhibitors reduce glucose uptake idependently of their effects on Akt. Biochem J 432: 191–197.
Um SH, D'Alessio D, Thoma G . (2006). Nutrient overload, insulin resistance and ribosomal protein S6 kinase 1, S6K1. Cell Metab 3: 393–402.
Wells V, Downward J, Malluci L . (2007). Functional inhibition of PI3K by the betaGBP molecule suppresses Ras-MAPK signaling to block cell proliferation. Oncogene 26: 7709–7714.
Acknowledgements
DLR received a mentor award from the American Diabetes Association. This work was funded by National Cancer Institute grant R01-5R01-CA128799. YF received grants from the Swiss National Science Foundation [PBBSB-120851 and PBBSB3-120851] and the Novartis Foundation, the Roche Research Foundation and the Oncosuisse Foundation. NVP-BKM120 and NVP-BEZ235 were supplied by Novartis Pharma (Basel, Switzerland) free of charge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gallagher, E., Fierz, Y., Vijayakumar, A. et al. Inhibiting PI3K reduces mammary tumor growth and induces hyperglycemia in a mouse model of insulin resistance and hyperinsulinemia. Oncogene 31, 3213–3222 (2012). https://doi.org/10.1038/onc.2011.495
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.495
Keywords
This article is cited by
-
Predictive, preventive, and personalized medicine in breast cancer: targeting the PI3K pathway
Journal of Translational Medicine (2024)
-
Exploring and exploiting the host cell autophagy during Mycobacterium tuberculosis infection
European Journal of Clinical Microbiology & Infectious Diseases (2023)
-
First-in-human phase Ia study of the PI3Kα inhibitor CYH33 in patients with solid tumors
Nature Communications (2022)
-
Insulin–PI3K signalling: an evolutionarily insulated metabolic driver of cancer
Nature Reviews Endocrinology (2020)
-
Hyperinsulinaemia in cancer
Nature Reviews Cancer (2020)