Abstract
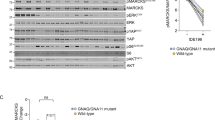
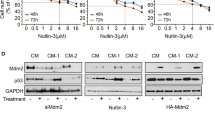
The prognosis of patients with uveal melanoma is poor. Because of the limited efficacy of current treatments, new therapeutic strategies need to be developed. Because p53 mutations are uncommon in uveal melanoma, reactivation of p53 may be used to achieve tumor regression. We investigated the use of combination therapies for intraocular melanoma, based on the p53 activators Nutlin-3 and reactivation of p53 and induction of tumor cell apoptosis (RITA) and the topoisomerase I inhibitor Topotecan. Nutlin-3 treatment induced p53-dependent growth inhibition in human uveal melanoma cell lines. The sensitivity to Nutlin-3 of the investigated cell lines did not correlate with basal Hdm2 or Hdmx levels. Nutlin-3 synergized with RITA and Topotecan to induce apoptosis in uveal melanoma cell lines and short-term cultures. Drug synergy correlated with enhanced induction of p53–Ser46 phosphorylation, which was attenuated by ATM inhibition. Nutlin-3 and Topotecan also significantly delayed tumor growth in vivo in a murine B16F10 model for ocular melanoma. Combination treatment appeared to inhibit tumor growth slightly more efficient than either drug alone. Nutlin-3, RITA and Topotecan lead to comparable p53 activation and growth inhibition under normoxia and hypoxia. Treatment with Nutlin-3 or RITA had no effect on HIF-1α induction by hypoxia, whereas the combination of these two drugs did inhibit hypoxia-induced HIF-1α. Also Topotecan, alone or in combination with Nutlin-3, reduced HIF-1α protein levels, suggesting that a certain level of DNA damage response is required for p53-mediated downregulation of HIF-1α. In conclusion, combination treatments based on small-molecule-induced p53 activation may have clinical potential for uveal melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM . (1998). Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 392: 405–408.
Augsburger JJ, Correa ZM, Shaikh AH . (2009). Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol 148: 119–127.
Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S et al. (2006). MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther 5: 2358–2365.
Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E et al. (1999). Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18: 6845–6854.
Chang AE, Karnell LH, Menck HR . (1998). The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84 836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83: 1664–1678.
Chou TC . (2006). Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58: 621–681.
Chou TC, Martin N . (2007). CompuSyn Software for Drug Combinations and for General Dose-Effect Analysis, and User's Guide 9-2-2007 ComboSyn, Inc.: Paramus, NJ.
Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de FM, Castano E et al. (2006). MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood 107: 4109–4114.
D'Orazi G, Cecchinelli B, Bruno T, Manni I, Higashimoto Y, Saito S et al. (2002). Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat Cell Biol 4: 11–19.
De Waard-Siebinga I, Blom DJ, Griffioen M, Schrier PI, Hoogendoorn E, Beverstock G et al. (1995). Establishment and characterization of an uveal-melanoma cell line. Int J Cancer 62: 155–161.
Enge M, Bao W, Hedstrom E, Jackson SP, Moumen A, Selivanova G. . (2009). MDM2-dependent downregulation of p21 and hnRNP K provides a switch between apoptosis and growth arrest induced by pharmacologically activated p53. Cancer Cell 15: 171–183.
Hu B, Gilkes DM, Farooqi B, Sebti SM, Chen J. . (2006). MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. J Biol Chem 281: 33030–33035.
Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M et al. (2004). Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 10: 1321–1328.
Kaluzova M, Kaluz S, Lerman MI, Stanbridge EJ. . (2004). DNA damage is a prerequisite for p53-mediated proteasomal degradation of HIF-1alpha in hypoxic cells and downregulation of the hypoxia marker carbonic anhydrase IX. Mol Cell Biol 24: 5757–5766.
Kivela T, Eskelin S, Kujala E. . (2006). Metastatic uveal melanoma. Int Ophthalmol Clin 46: 133–149.
Kodama M, Otsubo C, Hirota T, Yokota J, Enari M, Taya Y. . (2010). Requirement of ATM for rapid p53 phosphorylation at Ser46 without Ser/Thr-Gln sequences. Mol Cell Biol 30: 1620–1633.
Kojima K, Konopleva M, McQueen T, O'Brien S, Plunkett W, Andreeff M . (2006). Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood 108: 993–1000.
Krajewski M, Ozdowy P, D'Silva L, Rothweiler U, Holak TA . (2005). NMR indicates that the small molecule RITA does not block p53-MDM2 binding in vitro. Nat Med 11: 1135–1136.
Lam S, Lodder K, Teunisse AF, Rabelink MJ, Schutte M, Jochemsen AG . (2010). Role of Mdm4 in drug sensitivity of breast cancer cells. Oncogene 29: 2415–2426.
LaRusch GA, Jackson MW, Dunbar JD, Warren RS, Donner DB, Mayo LD . (2007). Nutlin3 blocks vascular endothelial growth factor induction by preventing the interaction between hypoxia inducible factor 1alpha and Hdm2. Cancer Res 67: 450–454.
Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C et al. (2006). Inactivation of the p53 pathway in retinoblastoma. Nature 444: 61–66.
Lee YM, Lim JH, Chun YS, Moon HE, Lee MK, Huang LE et al. (2009). Nutlin-3, an Hdm2 antagonist, inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated inactivation of HIF-1alpha. Carcinogenesis 30: 1768–1775.
Li Y, Yang DQ. . (2010). The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther 9: 113–125.
Ly LV, Baghat A, Versluis M, Jordanova ES, Luyten GP, van RN et al. (2010). In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol 185: 3481–3488.
Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY et al. (2005). Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 8: 99–110.
Patton JT, Mayo LD, Singhi AD, Gudkov AV, Stark GR, Jackson MW. . (2006). Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3. Cancer Res 66: 3169–3176.
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q et al. (2000). Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 14: 34–44.
Rinaldo C, Prodosmo A, Siepi F, Moncada A, Sacchi A, Selivanova G et al. (2009). HIPK2 regulation by MDM2 determines tumor cell response to the p53-reactivating drugs nutlin-3 and RITA. Cancer Res 69: 6241–6248.
Saha MN, Jiang H, Mukai A, Chang H. . (2010). RITA inhibits multiple myeloma cell growth through induction of p53-mediated caspase-dependent apoptosis and synergistically enhances nutlin-induced cytotoxic responses. Mol Cancer Ther 9: 3041–3051.
Saito S, Goodarzi AA, Higashimoto Y, Noda Y, Lees-Miller SP, Appella E et al. (2002). ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J Biol Chem 277: 12491–12494.
Tomicic MT, Christmann M, Kaina B. . (2010). Topotecan triggers apoptosis in p53-deficient cells by forcing degradation of XIAP and survivin thereby activating caspase-3-mediated Bid cleavage. J Pharmacol Exp Ther 332: 316–325.
Vassilev LT. . (2007). MDM2 inhibitors for cancer therapy. Trends Mol Med 13: 23–31.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. (2004). In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848.
Wade M, Wong ET, Tang M, Stommel JM, Wahl GM. . (2006). Hdmx modulates the outcome of p53 activation in human tumor cells. J Biol Chem 281: 33036–33044.
White JS, Choi S, Bakkenist CJ. . (2008). Irreversible chromosome damage accumulates rapidly in the absence of ATM kinase activity. Cell Cycle 7: 1277–1284.
Wynford-Thomas D, Blaydes J. . (1998). The influence of cell context on the selection pressure for p53 mutation in human cancer. Carcinogenesis 19: 29–36.
Yang J, Ahmed A, Poon E, Perusinghe N, de Haven BA, Box G et al. (2009). Small-molecule activation of p53 blocks hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in vivo and leads to tumor cell apoptosis in normoxia and hypoxia. Mol Cell Biol 29: 2243–2253.
Acknowledgements
We thank Dr PA van der Velden and M Versluis for the primer sets to detect the expression of the HIF target genes and the early cultures of primary uveal melanoma cells, Dr A Vertegaal for the antibodies detecting HIF-1α and HIF-2α proteins, Prof BR Ksander for providing the Mel cell lines, Dr G Selivanova for providing RITA and Dr A Levine for providing anti-Mdm2 antibody. This study was supported by grants from the Netherlands Organization for Scientific Research NWO (Mozaiek Grant 017.003.059) and by EC FP6 funding (contract 503576).
Disclaimer
This publication reflects the authors’ views and not necessarily those of the European Community. The EC is not liable for any use that may be made of the information contained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
de Lange, J., Ly, L., Lodder, K. et al. Synergistic growth inhibition based on small-molecule p53 activation as treatment for intraocular melanoma. Oncogene 31, 1105–1116 (2012). https://doi.org/10.1038/onc.2011.309
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.309
Keywords
This article is cited by
-
MDM2 inhibitors, nutlin-3a and navtemadelin, retain efficacy in human and mouse cancer cells cultured in hypoxia
Scientific Reports (2023)
-
Hypoxia-dependent drivers of melanoma progression
Journal of Experimental & Clinical Cancer Research (2021)
-
Tetra-substituted pyrrole derivatives act as potent activators of p53 in melanoma cells
Investigational New Drugs (2020)
-
Cell death-based treatment of neuroblastoma
Cell Death & Disease (2018)
-
Targeting MDMX and PKCδ to improve current uveal melanoma therapeutic strategies
Oncogenesis (2018)