Abstract
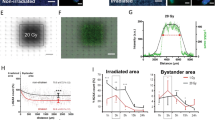
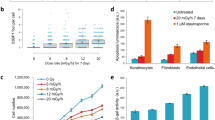
Ionizing radiation is a genotoxic agent and human carcinogen. Recent work has questioned long-held dogmas by showing that cancer-associated genetic alterations occur in cells and tissues not directly exposed to radiation, questioning the robustness of the current system of radiation risk assessment. In vitro, diverse mechanisms involving secreted soluble factors, gap junction intercellular communication (GJIC) and oxidative metabolism are proposed to mediate these indirect effects. In vivo, the mechanisms behind long-range ‘bystander’ responses remain largely unknown. Here, we investigate the role of GJIC in propagating radiation stress signals in vivo, and in mediating radiation-associated bystander tumorigenesis in mouse central nervous system using a mouse model in which intercellular communication is downregulated by targeted deletion of the connexin43 (Cx43) gene. We show that GJIC is critical for transmission of oncogenic radiation damage to the non-targeted cerebellum, and that a mechanism involving adenosine triphosphate release and upregulation of Cx43, the major GJIC constituent, regulates transduction of oncogenic damage to unirradiated tissues in vivo. Our data provide a novel hypothesis for transduction of distant bystander effects and suggest that the highly branched nervous system, similar to the vascular network, has an important role.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Amino M, Yoshioka K, Tanabe T, Tanaka E, Mori H, Furusawa Y et al. (2006). Heavy ion radiation up-regulates Cx43 and ameliorates arrhythmogenic substrates in hearts after myocardial infarction. Cardiovasc Res 72: 412–421.
Azzam EI, de Toledo SM, Gooding T, Little JB . (1998). Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res 150: 497–504.
Azzam EI, de Toledo SM, Little JB . (2003). Expression of connexin43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res 63: 7128–7135.
Azzam EI, de Toledo SM, Spitz DR, Little JB . (2002). Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res 62: 5436–5442.
BEIR VII (2006). Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. National Academies Press: Washington, DC.
Brenner DJ, Hall EJ . (2007). Computed tomography—an increasing source of radiation exposure. N Engl J Med 357: 2277–2284.
Chai Y, Hei TK . (2008). Radiation induced bystander effect in vivo. Acta Med Nagasaki 53: S65–S69.
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8: 752–758.
Evans WH, De Vuyst E, Leybaert L . (2006). The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J 397: 1–14.
Frantseva MV, Kokarovtseva L, Naus CG, Carlen PL, MacFabe D, Perez Velazquez JL . (2002). Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci 22: 644–653.
Huang L, Kim PM, Nickoloff JA, Morgan WF . (2007). Targeted and nontargeted effects of low-dose ionizing radiation on delayed genomic instability in human cells. Cancer Res 67: 1099–1104.
Kasper M, Traub O, Reimann T, Bjermer L, Grossmann H, Müller M et al. (1996). Upregulation of gap junction protein connexin43 in alveolar epithelial cells of rats with radiation-induced pulmonary fibrosis. Histochem Cell Biol 106: 419–424.
Liu K, Kasper M, Bierhaus A, Langer S, Müller M, Trott KR . (1997). Connexin 43 expression in normal and irradiated mouse skin. Radiat Res 147: 437–441.
Löbrich M, Jeggo PA . (2007). The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer 7: 861–869.
Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V et al. (2008). Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc Natl Acad Sci USA 105: 12445–12450.
Mothersill C, Seymour C . (2004). Radiation-induced bystander effects—implications for cancer. Nat Rev Cancer 4: 158–164.
Nagasawa H, Little JB . (1992). Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res 52: 6394–6396.
Ojima M, Furutani A, Ban N, Kai M . (2011). Persistence of DNA double-strand breaks in normal human cells induced by radiation-induced bystander effect. Radiat Res 175: 90–96.
Pazzaglia S, Tanori M, Mancuso M, Gessi M, Pasquali E, Leonardi S et al. (2006). Two-hit model for progression of medulloblastoma preneoplasia in Patched heterozygous mice. Oncogene 25: 5575–5580.
Qu Y, Dahl G . (2002). Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc Natl Acad Sci USA 99: 697–702.
Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC et al. (1995). Cardiac malformation in neonatal mice lacking connexin43. Science 267: 1831–1834.
Rogakou EP, Boon C, Redon C, Bonner WM . (1999). Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146: 905–916.
Rowitch DH, S-Jacques B, Lee SM, Flax JD, Snyder EY, McMahon AP . (1999). Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci 19: 8954–8965.
Schipke CG, Boucsein C, Ohlemeyer C, Kirchhoff F, Kettenmann H . (2002). Astrocyte Ca2+ waves trigger responses in microglial cells in brain slices. FASEB J 16: 255–257.
Sinclair CJ, LaRivière CG, Young JD, Cass CE, Baldwin SA, Parkinson FE . (2000). Purine uptake and release in rat C6 glioma cells: nucleoside transport and purine metabolism under ATP-depleting conditions. J Neurochem 75: 1528–1538.
Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM . (2007). Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res 67: 5872–5879.
Tsuda M, Ueno S, Inoue K . (1999). In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br J Pharmacol 127: 449–456.
Tsukimoto M, Homma T, Ohshima Y, Kojima S . (2010). Involvement of purinergic signaling in cellular response to gamma radiation. Radiat Res 173: 298–309.
Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P et al. (2004). P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med 10: 821–827.
Acknowledgements
This work was supported by Grant 10357 from the Associazione Italiana Ricerca sul Cancro (AIRC) to AS. We are grateful to Maria Pia Toni and Maria Pimpinella for assistance with dosimetry and Monte Carlo simulation. We also thank Alessandro Pannicelli for help with photographs and John Bechberger (University of British Columbia, Canada) for assistance with transfer of Cx43+/− mice.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Mancuso, M., Pasquali, E., Leonardi, S. et al. Role of connexin43 and ATP in long-range bystander radiation damage and oncogenesis in vivo. Oncogene 30, 4601–4608 (2011). https://doi.org/10.1038/onc.2011.176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.176
Keywords
This article is cited by
-
The roles of connexins and gap junctions in the progression of cancer
Cell Communication and Signaling (2023)
-
Cx43 channels and signaling via IP3/Ca2+, ATP, and ROS/NO propagate radiation-induced DNA damage to non-irradiated brain microvascular endothelial cells
Cell Death & Disease (2020)
-
Pharmacologically induced reversible hypometabolic state mitigates radiation induced lethality in mice
Scientific Reports (2017)
-
Connexins: substrates and regulators of autophagy
BMC Cell Biology (2016)
-
Upregulation of connexin43 contributes to PX-12-induced oxidative cell death
Tumor Biology (2016)