Abstract
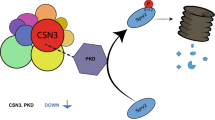
Steroid receptor co-activator-3 (SRC-3/AIB1) is an oncogene that is amplified and overexpressed in many human cancers. However, the molecular mechanisms that regulate ‘activated SRC-3 oncoprotein’ turnover during tumorigenesis remain to be elucidated. Here, we report that speckle-type POZ protein (SPOP), a cullin 3 (CUL3)-based ubiquitin ligase, is responsible for SRC-3 ubiquitination and proteolysis. SPOP interacts directly with an SRC-3 phospho-degron in a phosphorylation-dependent manner. Casein kinase Iɛ phosphorylates the S102 in this degron and promotes SPOP-dependent turnover of SRC-3. Short hairpin RNA knockdown and overexpression experiments substantiated that the SPOP/CUL3/Rbx1 ubiquitin ligase complex promotes SRC-3 turnover. A systematic analysis of the SPOP genomic locus revealed that a high percentage of genomic loss or loss of heterozygosity occurs at this locus in breast cancers. Furthermore, we demonstrate that restoration of SPOP expression inhibited SRC-3-mediated oncogenic signaling and tumorigenesis, thus positioning SPOP as a tumor suppressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY et al. (1997). AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277: 965–968.
Belandia B, Parker MG . (2000). Functional interaction between the p160 coactivator proteins and the transcriptional enhancer factor family of transcription factors. J Biol Chem 275: 30801–30805.
Bennett EJ, Rush J, Gygi SP, Harper JW . (2010). Dynamics of cullin-RING ubiquitin ligase network revealed by systematic quantitative proteomics. Cell 143: 951–965.
Bunce MW, Boronenkov IV, Anderson RA . (2008). Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J Biol Chem 283: 8678–8686.
Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L et al. (1997). Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90: 569–580.
Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R et al. (2009). Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev 23: 1910–1928.
Claiborn KC, Sachdeva MM, Cannon CE, Groff DN, Singer JD, Stoffers DA . (2010). Pcif1 modulates Pdx1 protein stability and pancreatic beta cell function and survival in mice. J Clin Invest 120: 3713–3721.
De Marchis L, Cropp C, Sheng ZM, Bargo S, Callahan R . (2004). Candidate target genes for loss of heterozygosity on human chromosome 17q21. Br J Cancer 90: 2384–2389.
Durocher F, Tonin P, Shattuck-Eidens D, Skolnick M, Narod SA, Simard J . (1996). Mutation analysis of the BRCA1 gene in 23 families with cases of cancer of the breast, ovary, and multiple other sites. J Med Genet 33: 814–819.
Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F et al. (2005). Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol 25: 2795–2807.
Furukawa M, He YJ, Borchers C, Xiong Y . (2003). Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat Cell Biol 5: 1001–1007.
Geyer R, Wee S, Anderson S, Yates J, Wolf DA . (2003). BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol Cell 12: 783–790.
Glass CK, Rosenfeld MG . (2000). The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14: 121–141.
Goel A, Janknecht R . (2004). Concerted activation of ETS protein ER81 by p160 coactivators, the acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J Biol Chem 279: 14909–14916.
Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M . (1994). Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science 264: 1455–1458.
Hernandez-Munoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E et al. (2005). Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proc Natl Acad Sci USA 102: 7635–7640.
Hershko A, Ciechanover A . (1998). The ubiquitin system. Annu Rev Biochem 67: 425–479.
Hong H, Kohli K, Trivedi A, Johnson DL, Stallcup MR . (1996). GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA 93: 4948–4952.
Jin J, Ang XL, Shirogane T, Wade Harper J . (2005). Identification of substrates for F-box proteins. Methods Enzymol 399: 287–309.
Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW . (2004). Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev 18: 2573–2580.
Kajiro M, Hirota R, Nakajima Y, Kawanowa K, So-ma K, Ito I et al. (2009). The ubiquitin ligase CHIP acts as an upstream regulator of oncogenic pathways. Nat Cell Biol 11: 312–319.
Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J . (2004). AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res 64: 1875–1885.
Kumar RPD, O'Malley BW . (2008). NR Coregulators and Human Diseases. World Scientific: Singapore; London, xi, 602 p.
Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH et al. (2006). BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. J Biol Chem 281: 12664–12672.
Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y et al. (2009). KEAP1 E3 Ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell 36: 131–140.
Lee SK, Kim HJ, Na SY, Kim TS, Choi HS, Im SY et al. (1998). Steroid receptor coactivator-1 coactivates activating protein-1-mediated transactivations through interaction with the c-Jun and c-Fos subunits. J Biol Chem 273: 16651–16654.
Lerebours F, Bertheau P, Bieche I, Driouch K, De The H, Hacene K et al. (2002). Evidence of chromosome regions and gene involvement in inflammatory breast cancer. Int J Cancer 102: 618–622.
Li C, Liang YY, Feng XH, Tsai SY, Tsai MJ, O'Malley BW . (2008a). Essential phosphatases and a phospho-degron are critical for regulation of SRC-3/AIB1 coactivator function and turnover. Mol Cell 31: 835–849.
Li C, Wu RC, Amazit L, Tsai SY, Tsai MJ, O'Malley BW . (2007a). Specific amino acid residues in the basic helix-loop-helix domain of SRC-3 are essential for its nuclear localization and proteasome-dependent turnover. Mol Cell Biol 27: 1296–1308.
Li H, Gomes PJ, Chen JD . (1997). RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA 94: 8479–8484.
Li LB, Louie MC, Chen HW, Zou JX . (2008b). Proto-oncogene ACTR/AIB1 promotes cancer cell invasion by up-regulating specific matrix metalloproteinase expression. Cancer Lett 261: 64–73.
Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW . (2007b). Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell 26: 831–842.
Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J et al. (2006). The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell 124: 381–392.
Liu J, Ghanim M, Xue L, Brown CD, Iossifov I, Angeletti C et al. (2009). Analysis of Drosophila segmentation network identifies a JNK pathway factor overexpressed in kidney cancer. Science 323: 1218–1222.
Louie MC, Zou JX, Rabinovich A, Chen HW . (2004). ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24: 5157–5171.
Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R et al. (2007). Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J 26: 1511–1521.
McKenna NJ, Lanz RB, O'Malley BW . (1999). Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20: 321–344.
McKenna NJ, O'Malley BW . (2002). Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108: 465–474.
Michaelson JS, Bader D, Kuo F, Kozak C, Leder P . (1999). Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev 13: 1918–1923.
O'Malley BW, Kumar R . (2009). Nuclear receptor coregulators in cancer biology. Cancer Res 69: 8217–8222.
Onate SA, Tsai SY, Tsai MJ, O'Malley BW . (1995). Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270: 1354–1357.
Orsetti B, Courjal F, Cuny M, Rodriguez C, Theillet C . (1999). 17q21–q25 aberrations in breast cancer: combined allelotyping and CGH analysis reveals 5 regions of allelic imbalance among which two correspond to DNA amplification. Oncogene 18: 6262–6270.
Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA et al. (2003). Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95: 353–361.
Petroski MD, Deshaies RJ . (2005). Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20.
Pickart CM . (2001). Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503–533.
Pintard L, Willis JH, Willems A, Johnson JL, Srayko M, Kurz T et al. (2003). The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature 425: 311–316.
Planas-Silva MD, Shang Y, Donaher JL, Brown M, Weinberg RA . (2001). AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res 61: 3858–3862.
Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H et al. (2008). The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol 28: 5937–5950.
Radford DM, Fair KL, Phillips NJ, Ritter JH, Steinbrueck T, Holt MS et al. (1995). Allelotyping of ductal carcinoma in situ of the breast: deletion of loci on 8p, 13q, 16q, 17p and 17q. Cancer Res 55: 3399–3405.
Rivers A, Gietzen KF, Vielhaber E, Virshup DM . (1998). Regulation of casein kinase I epsilon and casein kinase I delta by an in vivo futile phosphorylation cycle. J Biol Chem 273: 15980–15984.
Sakanaka C . (2002). Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. J Biochem 132: 697–703.
Shirogane T, Jin J, Ang XL, Harper JW . (2005). SCFbeta-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J Biol Chem 280: 26863–26872.
Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW . (1997). TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem 272: 27629–27634.
Tang J, Qu LK, Zhang J, Wang W, Michaelson JS, Degenhardt YY et al. (2006). Critical role for Daxx in regulating Mdm2. Nat Cell Biol 8: 855–862.
Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK et al. (1997). The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387: 677–684.
Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M et al. (2004). High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6: 263–274.
Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H . (1996). TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15: 3667–3675.
Wang Z, Rose DW, Hermanson O, Liu F, Herman T, Wu W et al. (2000). Regulation of somatic growth by the p160 coactivator p/CIP. Proc Natl Acad Sci USA 97: 13549–13554.
Welcker M, Clurman BE . (2008). FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 8: 83–93.
Werbajh S, Nojek I, Lanz R, Costas MA . (2000). RAC-3 is a NF-kappa B coactivator. FEBS Lett 485: 195–199.
Wu RC, Feng Q, Lonard DM, O'Malley BW . (2007). SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129: 1125–1140.
Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW . (2000). The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 97: 6379–6384.
Xu L, Wei Y, Reboul J, Vaglio P, Shin TH, Vidal M et al. (2003). BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature 425: 316–321.
Yan J, Erdem H, Li R, Cai Y, Ayala G, Ittmann M et al. (2008). Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res 68: 5460–5468.
Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ . (2006). Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res 66: 11039–11046.
Yi P, Feng Q, Amazit L, Lonard DM, Tsai SY, Tsai MJ et al. (2008). Atypical protein kinase C regulates dual pathways for degradation of the oncogenic coactivator SRC-3/AIB1. Mol Cell 29: 465–476.
Yu C, York B, Wang S, Feng Q, Xu J, O'Malley BW . (2007). An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell 25: 765–778.
Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai MJ . (2003). Role of the steroid receptor coactivator SRC-3 in cell growth. Mol Cell Biol 23: 7742–7755.
Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H et al. (2005). SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res 65: 7976–7983.
Zhuang M, Calabrese MF, Liu J, Waddell MB, Nourse A, Hammel M et al. (2009). Structures of SPOP-substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol Cell 36: 39–50.
Acknowledgements
We thank Ming-Jer Tsai and Sophia Y Tsai for their suggestions and Dr Kevin White (University of Chicago) for the monoclonal antibodies against SPOP. This work was supported by funding from State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute and grants (81072163) from National Natural Science Foundation of China (CL), and NIH HD-08188 and HD-07857 grants (BWO).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, C., Ao, J., Fu, J. et al. Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene 30, 4350–4364 (2011). https://doi.org/10.1038/onc.2011.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.151
Keywords
This article is cited by
-
SPOP mutations promote tumor immune escape in endometrial cancer via the IRF1–PD-L1 axis
Cell Death & Differentiation (2023)
-
BTB protein family and human breast cancer: signaling pathways and clinical progress
Journal of Cancer Research and Clinical Oncology (2023)
-
SPOP inhibits BRAF-dependent tumorigenesis through promoting non-degradative ubiquitination of BRAF
Cell & Bioscience (2022)
-
Neddylation inhibition induces glutamine uptake and metabolism by targeting CRL3SPOP E3 ligase in cancer cells
Nature Communications (2022)
-
The speckle-type POZ protein (SPOP) inhibits breast cancer malignancy by destabilizing TWIST1
Cell Death Discovery (2022)