Abstract
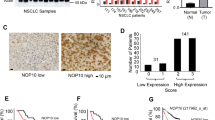
In an earlier study we showed that C10ORF97 (chromosome-10, open reading frame-97) was expressed in almost all of the tissues and cell lines tested, and that it inhibited the growth of seven tumor cell lines, including two lung carcinoma cell lines (A549 and PG). Here, we show that C10ORF97 is downregulated in non-small-cell lung cancer (NSCLC) tissue compared with normal lung tissue. Overexpression of C10ORF97 significantly suppressed human lung carcinoma A549 cell growth (proliferation and anchorage-independent growth in soft agar) and motility (migration and adhesion). This tumor-suppressive function of C10ORF97 was also verified in vivo. We further found that C10ORF97 caused G1 arrest of A549 cells and modulated the expression level of several cell-cycle regulators (such as CDK2, cyclin-E and p27). These effects of C10ORF97 were mediated by physical association between C10ORF97 and Jun-activating domain-binding protein-1 (JAB1), and blocking of JAB1-mediated translocation of p27 from the nucleus to the cytoplasm. Together, these results indicated that C10ORF97 functions as a novel tumor suppressor by modulating several key G1/S-regulatory proteins by interacting with JAB1. These findings led us to hypothesize that a single-nucleotide polymorphism (SNP) in the C10ORF97 gene that affects its expression might be associated with susceptibility to NSCLC. SNP216 C>T (rs2297882) in the C10ORF97 Kozak sequence was identified, and allele T of SNP216 suppressed C10ORF97 expression in vitro and in vivo. Furthermore, the TT genotype of SNP216 was associated with an increased risk of NSCLC (adjusted odds ratio=1.73 (95% confidence interval: 1.33–2.25), P=4.6 × 10–5). These data indicated that C10ORF97 is a tumor suppressor of NSCLC progression and C10ORF97-SNP216 may serve as a predictor of NSCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blain SW, Scher HI, Cordon-Cardo C, Koff A . (2003). p27 as a target for cancer therapeutics. Cancer Cell 3: 111–115.
Bloom J, Cross FR . (2007). Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol 8: 149–160.
Catzavelos C, Tsao MS, DeBoer G, Bhattacharya N, Shepherd FA, Slingerland JM . (1999). Reduced expression of the cell cycle inhibitor p27Kip1 in non-small cell lung carcinoma: a prognostic factor independent of Ras. Cancer Res 59: 684–688.
Chamovitz DA, Segal D . (2001). Jab1/CSN5 and the COP9 signalosome. A complex situation. EMBO Rep 2: 96–101.
Claret FX, Hibi M, Dhut S, Toda T, Karin M . (1996). A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature 383: 453–457.
Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG et al. (1997). Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 57: 3381–3385.
Frolova N, Edmonds MD, Bodenstine TM, Seitz R, Johnson MR, Feng R et al. (2009). A shift from nuclear to cytoplasmic breast cancer metastasis suppressor 1 expression is associated with highly proliferative estrogen receptor-negative breast cancers. Tumour Biol 30: 148–159.
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A et al. (2006). Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24: 4539–4544.
Hanahan D, Weinberg RA . (2000). The hallmarks of cancer. Cell 100: 57–70.
Hoffman PC, Mauer AM, Vokes EE . (2000). Lung cancer. Lancet 355: 479–485.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C et al. (2006). Cancer statistics, 2006. CA Cancer J Clin 56: 106–130.
Kastan MB, Bartek J . (2004). Cell-cycle checkpoints and cancer. Nature 432: 316–323.
Korbonits M, Chahal HS, Kaltsas G, Jordan S, Urmanova Y, Khalimova Z et al. (2002). Expression of phosphorylated p27(Kip1) protein and Jun activation domain-binding protein 1 in human pituitary tumors. J Clin Endocrinol Metab 87: 2635–2643.
Kouvaraki MA, Rassidakis GZ, Tian L, Kumar R, Kittas C, Claret FX . (2003). Jun activation domain-binding protein 1 expression in breast cancer inversely correlates with the cell cycle inhibitor p27(Kip1). Cancer Res 63: 2977–2981.
Liu B, Liu Y, Chen J, Wei Z, Yu H, Zhen Y et al. (2002). CARP is a novel caspase recruitment domain containing proapoptotic protein. Biochem Biophys Res Commun 293: 1396–1404.
Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC et al. (1999). p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 154: 313–323.
Malumbres M, Barbacid M . (2007). Cell cycle kinases in cancer. Curr Opin Genet Dev 17: 60–65.
Masciullo V, Susini T, Zamparelli A, Bovicelli A, Minimo C, Massi D et al. (2003). Frequent loss of expression of the cyclin-dependent kinase inhibitor p27(Kip1) in estrogen-related endometrial adenocarcinomas. Clin Cancer Res 9: 5332–5338.
Massague J . (2004). G1 cell-cycle control and cancer. Nature 432: 298–306.
Meyerson M, Franklin WA, Kelley MJ . (2004). Molecular classification and molecular genetics of human lung cancers. Semin Oncol 31: 4–19.
Osoegawa A, Yoshino I, Kometani T, Yamaguchi M, Kameyama T, Yohena T et al. (2006). Overexpression of Jun activation domain-binding protein 1 in nonsmall cell lung cancer and its significance in p27 expression and clinical features. Cancer 107: 154–161.
Parkin DM, Bray F, Ferlay J, Pisani P . (2005). Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108.
Rassidakis GZ, Claret FX, Lai R, Zhang Q, Sarris AH, McDonnell TJ et al. (2003). Expression of p27(Kip1) and c-Jun activation binding protein 1 are inversely correlated in systemic anaplastic large cell lymphoma. Clin Cancer Res 9: 1121–1128.
Sato M, Shames DS, Gazdar AF, Minna JD . (2007). A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol 2: 327–343.
Shaulian E, Karin M . (2002). AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136.
Sherr CJ . (2000). The Pezcoller lecture: cancer cell cycles revisited. Cancer Res 60: 3689–3695.
Sheer CJ, Roberts JM . (1999). CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512.
Shintani S, Li C, Mihara M, Hino S, Nakashiro K, Hamakawa H . (2003). Skp2 and Jab1 expression are associated with inverse expression of p27(KIP1) and poor prognosis in oral squamous cell carcinomas. Oncology 65: 355–362.
Slingerland J, Pagano M . (2000). Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 183: 10–17.
Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tai Y et al. (2001). Jab1 expression is associated with inverse expression of p27(kip1) and poor prognosis in epithelial ovarian tumors. Clin Cancer Res 7: 4130–4135.
Tomoda K, Kubota Y, Arata Y, Mori S, Maeda M, Tanaka T et al. (2002). The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem 277: 2302–2310.
Tomoda K, Kubota Y, Kato J . (1999). Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398: 160–165.
Tsukamoto S, Sugio K, Sakada T, Ushijima C, Yamazaki K, Sugimachi K . (2001). Reduced expression of cell-cycle regulator p27(Kip1) correlates with a shortened survival in non-small cell lung cancer. Lung Cancer 34: 83–90.
Weinstein IB, Joe AK . (2006). Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol 3: 448–457.
Wu FY, Wang SE, Sanders ME, Shin I, Rojo F, Baselga J et al. (2006). Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res 66: 2162–2172.
Viglietto G, Motti ML, Fusco A . (2002). Understanding p27(kip1) deregulation in cancer: downregulation or mislocalization. Cell Cycle 1: 394–400.
Acknowledgements
We thank Professor Gu Xiaocheng and Zheng Xiaofeng (Department of Biochemistry and Molecular Biology, College of Life Sciences, Peking University, Beijing, China) for preparation and purification of the C10ORF97 protein. Sources of support: This study was supported by the National High Technology Research and Development Program of China (863 Program, No. 2006AA02Z477 to RH), the National Natural Science Foundation of China (No. 30500199 to JC and No. 30900809 to YS) and the Foundation of Beijing Municipal Committee of Science and Technology (No. D0905001040631 to JJ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Shi, Y., Chen, J., Li, Z. et al. C10ORF97 is a novel tumor-suppressor gene of non-small-cell lung cancer and a functional variant of this gene increases the risk of non-small-cell lung cancer. Oncogene 30, 4107–4117 (2011). https://doi.org/10.1038/onc.2011.116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.116
Keywords
This article is cited by
-
Characterization of universal features of partially methylated domains across tissues and species
Epigenetics & Chromatin (2020)