Abstract
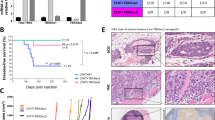
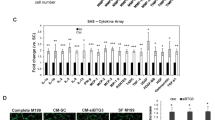
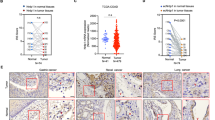
ADAMTS (a disintegrin and metalloproteinase domain with thrombospondin motifs) constitute a family of endopeptidases related to matrix metalloproteinases. These proteases have been largely implicated in tissue remodeling and angiogenesis associated with physiological and pathological processes. To elucidate the in vivo functions of ADAMTS-12, we have generated a knockout mouse strain (Adamts12−/−) in which Adamts12 gene was deleted. The mutant mice had normal gestations and no apparent defects in growth, life span and fertility. By applying three different in vivo models of angiogenesis (malignant keratinocyte transplantation, Matrigel plug and aortic ring assays) to Adamts12−/− mice, we provide evidence for a protective effect of this host enzyme toward angiogenesis and cancer progression. In the absence of Adamts-12, both the angiogenic response and tumor invasion into host tissue were increased. Complementing results were obtained by using medium conditioned by cells overexpressing human ADAMTS-12, which inhibited vessel outgrowth in the aortic ring assay. This angioinhibitory effect of ADAMTS-12 was independent of its enzymatic activity as a mutated inactive form of the enzyme was similarly efficient in inhibiting endothelial cell sprouting in the aortic ring assay than the wild-type form. Altogether, our results show that ADAMTS-12 displays antiangiogenic properties and protect the host toward tumor progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bai XH, Wang DW, Luan Y, Yu XP, Liu CJ . (2009). Regulation of chondrocyte differentiation by ADAMTS-12 metalloproteinase depends on its enzymatic activity. Cell Mol Life Sci 66: 667–680.
Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A et al. (2003). Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet 35: 252–257.
Berndt S, Bruyere F, Jost M, Edwards DR, Noel A . (2008). In vitro and in vivo models of angiogenesis to dissect MMP functions. In Edwards DR, Hoyer-Hansen G, Blasi F, Sloane BF (eds). The Cancer Degradome: Proteases and Cancer Biology. Springer: New-York, 305–325.
Berndt S, Perrier DS, Blacher S, Pequeux C, Lorquet S, Munaut C et al. (2006). Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J 20: 2630–2632.
Blacher S, Jost M, Melen-Lamalle L, Lund LR, Romer J, Foidart JM et al. (2008). Quantification of in vivo tumor invasion and vascularization by computerized image analysis. Microvasc Res 75: 169–178.
Cal S, Arguelles JM, Fernandez PL, Lopez-Otin C . (2001). Identification, characterization, and intracellular processing of ADAM-TS12, a novel human disintegrin with a complex structural organization involving multiple thrombospondin-1 repeats. J Biol Chem 276: 17932–17940.
Cauwe B, Van den Steen PE, Opdenakker G . (2007). The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol 42: 113–185.
Dunn JR, Reed JE, du Plessis DG, Shaw EJ, Reeves P, Gee AL et al. (2006). Expression of ADAMTS-8, a secreted protease with antiangiogenic properties, is downregulated in brain tumours. Br J Cancer 94: 1186–1193.
Egeblad M, Werb Z . (2002). New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174.
Fusenig NE, Breitkreutz D, Dzarlieva RT, Boukamp P, Bohnert A, Tilgen W . (1983). Growth and differentiation characteristics of transformed keratinocytes from mouse and human skin in vitro and in vivo. J Invest Dermatol 81: 168s–175s.
Iruela-Arispe ML, Carpizo D, Luque A . (2003). ADAMTS1: a matrix metalloprotease with angioinhibitory properties. Ann N Y Acad Sci 995: 183–190.
Jost M, Folgueras AR, Frerart F, Pendas A, Blacher S, Houard X et al. (2006). Earlier onset of tumoral angiogenesis in matrix metalloproteinase-19-deficient mice. Cancer Res 66: 5234–5241.
Jost M, Maillard C, Lecomte J, Lambert V, Tjwa M, Blaise P et al. (2007). Tumoral and choroidal vascularization: differential cellular mechanisms involving plasminogen activator inhibitor type I. Am J Pathol 171: 1369–1380.
Jost M, Vosseler S, Blacher S, Fusenig NE, Mueller MM, Noel A . (2008). The surface transplantation model to study the tumor-host interface. In Edwards DR, Hoyer-Hansen G, Blasi F, Sloane BF (eds). The Cancer Degradome: Proteases and Cancer Biology. Springer: New York, pp 327–342.
Kurz T, Hoffjan S, Hayes MG, Schneider D, Nicolae R, Heinzmann A et al. (2006). Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci. J Allergy Clin Immunol 118: 396–402.
Lambert V, Wielockx B, Munaut C, Galopin C, Jost M, Itoh T et al. (2003). MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J 17: 2290–2292.
Liu YJ, Xu Y, Yu Q . (2006). Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene 25: 2452–2467.
Llamazares M, Obaya AJ, Moncada-Pazos A, Heljasvaara R, Espada J, Lopez-Otin C et al. (2007). The ADAMTS12 metalloproteinase exhibits anti-tumorigenic properties through modulation of the Ras-dependent ERK signalling pathway. J Cell Sci 120: 3544–3552.
Lopez-Otin C, Matrisian LM . (2007). Emerging roles of proteases in tumour suppression. Nat Rev Cancer 7: 800–808.
Luque A, Carpizo DR, Iruela-Arispe ML . (2003). ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem 278: 23656–23665.
Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S et al. (2002). Mouse aortic ring assay: a new approach of the molecular genetics of angiogenesis. Biol Proced Online 4: 24–31.
Moncada-Pazos A, Obaya AJ, Fraga MF, Viloria CG, Capella G, Gausachs M et al. (2009). The ADAMTS12 metalloprotease gene is epigenetically silenced in tumor cells and transcriptionally activated in the stroma during progression of colon cancer. J Cell Sci 122: 2906–2913.
Mueller MM, Fusenig NE . (2004). Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4: 839–849.
Noel A, Jost M, Maquoi E . (2008). Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol 19: 52–60.
Nyberg P, Salo T, Kalluri R . (2008). Tumor microenvironment and angiogenesis. Front Biosci 13: 6537–6553.
Overall CM, Blobel CP . (2007). In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol 8: 245–257.
Porter S, Clark IM, Kevorkian L, Edwards DR . (2005). The ADAMTS metalloproteinases. Biochem J 386: 15–27.
Porter S, Scott SD, Sassoon EM, Williams MR, Jones JL, Girling AC et al. (2004). Dysregulated expression of adamalysin-thrombospondin genes in human breast carcinoma. Clin Cancer Res 10: 2429–2440.
Rocks N, Paulissen G, El Hour M, Quesada F, Crahay C, Gueders M et al. (2008). Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie 90: 369–379.
Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M et al. (1999). METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 274: 23349–23357.
Acknowledgements
The authors acknowledge F Olivier, G Roland and L Volders for their excellent technical assistance. This work was supported by grants from Ministerio de Ciencia e Innovación, Fundación M. Botin (Spain), the FP7-HEALTH-2007-A—Project No. 201279 ‘MICROENVIMET’, the Fonds de la Recherche Scientifique-FNRS (FRS-FNRS, Belgium), the Foundation against Cancer (foundation of public interest, Belgium), the DGTRE from the SPW (Région Wallonne, Belgium), the Interuniversity Attraction Poles Programme—Belgian Science Policy (Brussels, Belgium). MEH, AM, JD, LH and FE are recipients of grants from the Fonds de la Recherche Scientifique (FRS-FNRS, Belgium).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
El Hour, M., Moncada-Pazos, A., Blacher, S. et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene 29, 3025–3032 (2010). https://doi.org/10.1038/onc.2010.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.49
Keywords
This article is cited by
-
Comprehensive Analysis of ADAMTS Gene Family in Renal Clear Cell Carcinoma and ADAMTS10 Research Combining Magnetic Resonance Imaging
Molecular Biotechnology (2023)
-
Identification of relevant genetic alterations in cancer using topological data analysis
Nature Communications (2020)
-
ADAMTS Sol narae cleaves extracellular Wingless to generate a novel active form that regulates cell proliferation in Drosophila
Cell Death & Disease (2019)
-
ADAMTS-18 in the host tissues exerts little effect on breast tumor progress in a murine 4T1 breast cancer model
Journal of Negative Results in BioMedicine (2016)
-
The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family
Genome Biology (2015)