Abstract
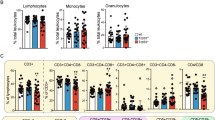
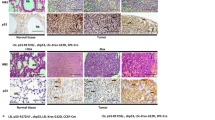
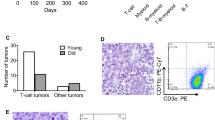
As a transcription factor, the critical tumor suppressor, p53, directly regulates the transcription of hundreds of genes, leading to cell-cycle arrest, apoptosis, cellular senescence and differentiation. Although it has been assumed that p53 transcription activity is critical for tumor suppression, this assumption has been increasingly contested by recent findings of transcription-independent roles of p53 in apoptosis as well as findings that none of the mutant mice lacking important p53 transcription targets are cancer prone. On the basis of previous findings that p53 transcription activity is abolished in p53QS (Leu25Trp26 to Gln25Ser26) knock-in mouse cells after DNA damage, to determine the importance of transcription activity of p53 in tumor suppression, we generated knock-in mice that can conditionally express p53QS protein in a Cre-dependent manner. By breeding the knock-in mice with Lck-Cre transgenic mice that specifically express Cre in thymocytes, we show that p53-dependent suppression of thymic lymphomas is abolished in thymocytes expressing high levels of p53QS protein. In addition, p53QS protein is accumulated in some of the thymic tumors. Therefore, p53 transcription activity induced by DNA damage is required for tumor suppression. Together with the findings that the disruption of various p53-dependent functions individually fails to promote cancer, our findings indicate that various transcription-dependent functions of p53 must collaborate to efficiently suppress tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ . (1995). Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377: 552–557.
Chao C, Hergenhahn M, Kaeser MD, Wu Z, Saito S, Iggo R et al. (2003). Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem 278: 41028–41033.
Chao C, Herr D, Chun J, Xu Y . (2006a). Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. EMBO J 25: 2615–2622.
Chao C, Saito S, Kang J, Anderson CW, Appella E, Xu Y . (2000). p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO J 19: 4967–4975.
Chao C, Wu Z, Mazur SJ, Borges H, Rossi M, Lin T et al. (2006b). Acetylation of mouse p53 at lysine 317 negatively regulates p53 apoptotic activities after DNA damage. Mol Cell Biol 26: 6859–6869.
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M et al. (2004). Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303: 1010–1014.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P . (1995). Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82: 675–684.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery Jr CA, Butel JS et al. (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221.
Inlay M, Alt FW, Baltimore D, Xu Y . (2002). Essential roles of the [kappa] light chain intronic enhancer and 3[prime] enhancer in [kappa] rearrangement and demethylation. Nat Immunol 3: 463–468.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. (1994). Tumor spectrum analysis in p53-mutant mice. Curr Biol 4: 1–7.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. (2003). Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4: 321–328.
Johnson TM, Hammond EM, Giaccia A, Attardi LD . (2005). The p53QS transactivation-deficient mutant shows stress-specific apoptotic activity and induces embryonic lethality. Nat Genet 37: 145–152.
Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A . (2001). Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 29: 418–425.
Ko LJ, Prives C . (1996). p53: puzzle and paradigm. Genes Dev 10: 1054–1072.
Kortlever RM, Higgins PJ, Bernards R . (2006). Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 8: 877–884.
Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM et al. (2004). Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119: 861–872.
Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW et al. (2001). A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15: 763–774.
Lin J, Chen J, Elenbaas B, Levine AJ . (1994). Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev 8: 1235–1246.
Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E et al. (2005). p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 7: 165–171.
Michael D, Oren M . (2002). The p53 and Mdm2 families in cancer. Curr Opin Genet Dev 12: 53–59.
Moll UM, Wolff S, Speidel D, Deppert W . (2005). Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol 17: 631–636.
Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT et al. (2004). Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119: 847–860.
Oren M . (2003). Decision making by p53: life, death and cancer. Cell Death Differ 10: 431–442.
Schuler M, Green DR . (2005). Transcription, apoptosis and p53: catch-22. Trends Genet 21: 182–187.
Song H, Hollstein M, Xu Y . (2007). p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol 9: 573–580.
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ et al. (2003). p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302: 1036–1038.
Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T et al. (2006). A global map of p53 transcription-factor binding sites in the human genome. Cell 124: 207–219.
Xu Y . (2005). A new role for p53 in maintaining genetic stability in embryonic stem cells. Cell Cycle 4: 363–364.
Acknowledgements
We thank T Lin and C Chao for their help in constructing the p53QS mice. This work was supported by a NIH grant (R01 CA94254) to YX.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaidarenko, O., Xu, Y. Transcription activity is required for p53-dependent tumor suppression. Oncogene 28, 4397–4401 (2009). https://doi.org/10.1038/onc.2009.290
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.290
Keywords
This article is cited by
-
Altered pathways and targeted therapy in double hit lymphoma
Journal of Hematology & Oncology (2022)