Abstract
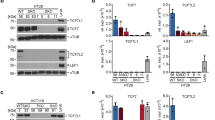
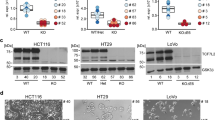
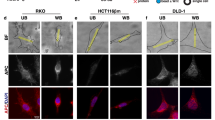
Constitutive activation of the Wnt/β-catenin pathway has been implicated as the primary cause of colon cancer. However, the major transducers of Wnt signaling in the intestine, T-cell factor 1 (TCF-1) and TCF-4, have opposing functions. Knockout of TCF-4 suppresses growth and maintenance of crypt stem cells, whereas knockout of TCF-1 leads to adenomas. These phenotypes suggest that TCF-4 is Wnt-promoting, whereas TCF-1 acts like a tumor suppressor. Our study of TCF expression in human colon crypts reveals a mechanistic basis for this paradox. In normal colon cells, a dominant-negative isoform of TCF-1 (dnTCF-1) is expressed that is equally distributed between nuclear and cytoplasmic compartments. In colon cancer cells, TCF-1 is predominantly cytoplasmic. Localization is because of active nuclear export and is directed by an autocrine-acting Wnt ligand that requires Ca2+/calmodulin-dependent kinase II (CaMKII) activity for secretion and a downstream step in the export pathway. TCF-4 remains nuclear; its unopposed activity is accompanied by downregulation of dnTCF-1 and increased expression of full-length isoforms. Thus, the dnTCF-1 and TCF-4 balance is corrupted in cancer by two mechanisms, a Wnt/CaMKII kinase signal for nuclear export and decreased dnTCF-1 expression. We propose that dnTCF-1 provides homeostatic regulation of Wnt signaling and growth in normal colon, and the alterations in nuclear export and promoter usage contribute to aberrant Wnt activity in colon cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Arce L, Yokoyama NN, Waterman ML . (2006). Diversity of LEF/TCF action in development and disease. Oncogene 25: 7492–7504.
Atcha FA, Syed A, Wu B, Hoverter NP, Yokoyama NN, Ting JH et al. (2007). A unique DNA binding domain converts T-cell factors into strong Wnt effectors. Mol Cell Biol 27: 8352–8363.
Bienz M, Clevers H . (2000). Linking colorectal cancer to Wnt signaling. Cell 103: 311–320.
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J . (2008). Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121: 737–746.
Chang MV, Chang JL, Gangopadhyay A, Shearer A, Cadigan KM . (2008). Activation of wingless targets requires bipartite recognition of DNA by TCF. Curr Biol 18: 1877–1881.
Clevers H . (2006). Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480.
Fornerod M, Ohno M, Yoshida M, Mattaj IW . (1997). CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90: 1051–1060.
Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M et al. (1997). CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390: 308–311.
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C . (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362.
Griffith LC . (2008). CaMKII: new tricks for an old dog. Cell 133: 397–399.
Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S et al. (2008). Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol 28: 2732–2744.
Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J et al. (2001). Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 28: 53–57.
Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M et al. (2003a). The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 23: 131–139.
Ishitani T, Ninomiya-Tsuji J, Matsumoto K . (2003b). Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol 23: 1379–1389.
Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N et al. (1999). The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399: 798–802.
Kinzler KW, Vogelstein B . (1996). Lessons from hereditary colorectal cancer. Cell 87: 159–170.
Klaus A, Birchmeier W . (2008). Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8: 387–398.
Kohn AD, Moon RT . (2005). Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 38: 439–446.
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ et al. (1998). Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383.
Lo MC, Gay F, Odom R, Shi Y, Lin R . (2004). Phosphorylation by the beta-catenin/MAPK complex promotes 14-3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell 117: 95–106.
Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y et al. (1992). Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA 89: 4452–4456.
Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R et al. (1999). Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285: 1923–1926.
Sievers S, Fritzsch C, Grzegorczyk M, Kuhnen C, Muller O . (2006). Absolute beta-catenin concentrations in Wnt pathway-stimulated and non-stimulated cells. Biomarkers 11: 270–278.
Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T et al. (1991). The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181: 968–975.
Tang W, Dodge M, Gundapaneni D, Michnoff C, Roth M, Lum L . (2008). A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc Natl Acad Sci USA 105: 9697–9702.
Van de Wetering M, Castrop J, Korinek V, Clevers H . (1996). Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol Cell Biol 16: 745–752.
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A et al. (2002). The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250.
Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M et al. (2007). The intestinal Wnt/TCF signature. Gastroenterology 132: 628–632.
van Noort M, Clevers H . (2002). TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev Biol 244: 1–8.
Yamada M, Ohnishi J, Ohkawara B, Iemura S, Satoh K, Hyodo-Miura J et al. (2006). NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF). J Biol Chem 281: 20749–20760.
Acknowledgements
We specially thank Didi Mwengela, Amanda Chamberlain, Candice Solomon and David Hernandez for help in cloning and sequencing TCF-1 cDNA. We also thank Eric J Stanbridge for critical reading of the manuscript, Michelle Digman for help with confocal imaging, Tohru Ishitani, Bang Hoang and Randall T Moon for reagents and advice. This work was supported by NIH grants RO1 HD36081 and RO1 HD36049 to JLM, NIH T32 CA113265, CA096878 and CA108697 to MLW, R21 DK071591 to RAE and NIH grant CA-82450 to RFH. This work was made possible, in part, through access to the confocal facility of the Optical Biology Shared Resource of the Cancer Center Support Grant (CA-62203) at the University of California, Irvine.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Najdi, R., Syed, A., Arce, L. et al. A Wnt kinase network alters nuclear localization of TCF-1 in colon cancer. Oncogene 28, 4133–4146 (2009). https://doi.org/10.1038/onc.2009.271
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.271
Keywords
This article is cited by
-
β-catenin/TCF activity regulates IGF-1R tyrosine kinase inhibitor sensitivity in colon cancer
Oncogene (2018)
-
Gene expression profiles reveal key genes for early diagnosis and treatment of adamantinomatous craniopharyngioma
Cancer Gene Therapy (2018)
-
Roles of transcriptional factor 7 in production of inflammatory factors for lung diseases
Journal of Translational Medicine (2015)
-
A reverse-engineering approach to dissect post-translational modulators of transcription factor’s activity from transcriptional data
BMC Bioinformatics (2015)
-
SKLB-677, an FLT3 and Wnt/β-catenin signaling inhibitor, displays potent activity in models of FLT3-driven AML
Scientific Reports (2015)