Abstract
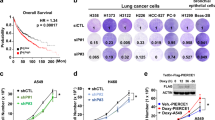
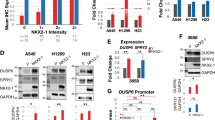
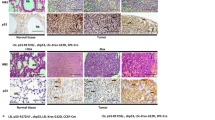
Reduced expression of the CDK inhibitor p27Kip1 (p27) in human lung cancer correlates with tumor aggressiveness and poor prognosis. However, the regulation of p27 expression and the role of p27 during lung cancer are poorly understood. Urethane-induced lung tumors in mice frequently harbor mutations in the Kras oncogene, and in this study, we use this model to address the regulation of p27 during tumorigenesis. The Ras effector Akt is known to regulate p27 mRNA abundance by phosphorylating and inactivating the FOXO transcription factors. Phosphorylated Akt and FOXO proteins were both increased in lung tumors, correlating with a reduction in p27 mRNA transcript. Akt also directly phosphorylates p27 and regulates its nuclear/cytoplasmic localization. Tumors showed a reduced nuclear/cytoplasmic ratio of p27 protein, together with an increase in phosphorylated Thr197 p27 in the cytoplasmic pool. Treatment of lung tumor-bearing mice with the phosphoinositol-3 kinase inhibitor LY294002 induced a rapid decrease in phosphorylated Akt and phosphorylated p27, concomitant with an increase in nuclear p27. Germline p27 deficiency accelerated both the growth and malignant progression of urethane-induced lung tumors, and did so in a cell autonomous manner, confirming a causal role of p27 in tumor suppression. These results show that p27 is a potent barrier to the growth and malignant progression of Kras-initiated lung tumors. Further, the reduction of nuclear p27 in tumors is mediated by oncogene signaling pathways, which can be reversed by pharmacological agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Baldassarre G, Belletti B, Nicoloso MS, Schiappacassi M, Vecchione A, Spessotto P et al. (2005). p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 7: 51–63.
Besson A, Assoian RK, Roberts JM . (2004a). Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nat Rev Cancer 4: 948–955.
Besson A, Gurian-West M, Chen X, Kelly-Spratt KS, Kemp CJ, Roberts JM . (2006). A pathway in quiescent cells that controls p27/Kip1 stability, subcellular localization, and tumor suppression. Genes Dev 20: 47–64.
Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM . (2004b). p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev 18: 862–876.
Blain SW, Massague J . (2002). Breast cancer banishes p27/Kip1 from the nucleus. Nat Med 8: 1076–1078.
Bloom J, Pagano M. . (2003). Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol 13: 41–47.
Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ et al. (2002). A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J 21: 3390–3401.
Busse D, Doughty RS, Ramsey TT, Russell WE, Price JO, Flanagan WM et al. (2000). Reversible G(1) arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27(KIP1) independent of MAPK activity. J Biol Chem 275: 6987–6995.
Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich Jr V, Chao MV, Koff A . (1997). Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev 11: 2335–2346.
Catzavelos C, Tsao MS, DeBoer G, Bhattacharya N, Shepherd FA, Slingerland JM . (1999). Reduced expression of the cell cycle inhibitor p27Kip1 in non-small cell lung carcinoma: a prognostic factor independent of Ras. Cancer Res 59: 684–688.
Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S et al. (2007). p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128: 281–294.
Chu IM, Hengst L, Slingerland JM . (2008). The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 8: 253–267.
Connor MK, Kotchetkov R, Cariou S, Resch A, Lupetti R, Beniston RG et al. (2003). CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell 14: 201–213.
Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW et al. (2000). Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 20: 9138–9148.
Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K et al. (2008). Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455: 1069–1075.
Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ . (1998). The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396: 177–180.
Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E et al. (1996). A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27-deficient mice. Cell 85: 733–744.
Fujita N, Sato S, Tsuruo T . (2003). Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem 278: 49254–49260.
Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB et al. (2007). Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128: 269–280.
Inui N, Kitagawa K, Miwa S, Hattori T, Chida K, Nakamura H et al. (2003). High expression of Cks1 in human non-small cell lung carcinomas. Biochem Biophys Res Commun 303: 978–984.
Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S et al. (2004). Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol 6: 1229–1235.
Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T et al. (1995). Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 55: 2266–2269.
Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M et al. (1996). Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85: 721–732.
Kossatz U, Vervoorts J, Nickeleit I, Sundberg HA, Arthur JS, Manns MP et al. (2006). C-terminal phosphorylation controls the stability and function of p27kip1. EMBO J 25: 5159–5170.
Kotake Y, Nakayama K, Ishida N, Nakayama KI . (2005). Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation. J Biol Chem 280: 1095–1102.
Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K et al. (2002). PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8: 1153–1160.
Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF et al. (1997). Increased proteasome-dependent degradation of the cyclin- dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 3: 231–234.
Malkinson AM . (2001). Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer 32: 265–279.
Martins CP, Brown-Swigart L, Evan GI . (2006). Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127: 1323–1334.
McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF . (2003). Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol 23: 216–228.
Medema RH, Kops GJ, Bos JL, Burgering BM . (2000). AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404: 782–787.
Motti ML, Califano D, Troncone G, De MC, Migliaccio I, Palmieri E et al. (2005). Complex regulation of the cyclin-dependent kinase inhibitor p27kip1 in thyroid cancer cells by the PI3K/AKT pathway: regulation of p27kip1 expression and localization. Am J Pathol 166: 737–749.
Motti ML, De MC, Califano D, Fusco A, Viglietto G . (2004). Akt-dependent T198 phosphorylation of cyclin-dependent kinase inhibitor p27kip1 in breast cancer. Cell Cycle 3: 1074–1080.
Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N et al. (1996). Mice lacking p27 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85: 707–720.
Philipp J, Vo K, Gurley KE, Seidel K, Kemp CJ . (1999). Tumor suppression by p27/Kip1 and p21/Cip1 during chemically induced skin carcinogenesis. Oncogene 18: 4689–4698.
Philipp-Staheli J, Kim KH, Liggitt D, Gurley KE, Longton G, Kemp CJ . (2004). Distinct roles for p53, p27Kip1, and p21Cip1 during tumor development. Oncogene 23: 905–913.
Philipp-Staheli J, Kim KH, Payne SR, Gurley KE, Liggitt D, Longton G et al. (2002). Pathway-specific tumor suppression. Reduction of p27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer Cell 1: 355–368.
Philipp-Staheli J, Payne SR, Kemp CJ . (2001). p27(Kip1): regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp Cell Res 264: 148–168.
Ponce-Castaneda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K et al. (1995). p27Kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res 55: 1211–1214.
Sa G, Stacey DW . (2004). P27 expression is regulated by separate signaling pathways, downstream of Ras, in each cell cycle phase. Exp Cell Res 300: 427–439.
Schiappacassi M, Lovat F, Canzonieri V, Belletti B, Berton S, Di SD et al. (2008). p27Kip1 expression inhibits glioblastoma growth, invasion, and tumor-induced neoangiogenesis. Mol Cancer Ther 7: 1164–1175.
Schreiber E, Matthias P, Muller MM, Schaffner W . (1989). Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res 17: 6419.
Shaffer DR, Viale A, Ishiwata R, Leversha M, Olgac S, Manova K et al. (2005). Evidence for a p27 tumor suppressive function independent of its role regulating cell proliferation in the prostate. Proc Natl Acad Sci USA 102: 210–215.
Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE . (1997). Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev 11: 1464–1478.
Shin I, Rotty J, Wu FY, Arteaga CL . (2005). Phosphorylation of p27Kip1 at Thr-157 interferes with its association with importin alpha during G1 and prevents nuclear re-entry. J Biol Chem 280: 6055–6063.
Singhal S, Vachani A, ntin-Ozerkis D, Kaiser LR, Albelda SM . (2005). Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res 11: 3974–3986.
Susaki E, Nakayama KI . (2007). Multiple mechanisms for p27(Kip1) translocation and degradation. Cell Cycle 6: 3015–3020.
Tomoda K, Kubota Y, Kato J . (1999). Degradation of the cyclin-dependent-kinase inhibitor p27(Kip1) is instigated by Jab1. Nature 398: 160–165.
Tong W, Kiyokawa H, Soos TJ, Park MS, Soares VC, Manova K et al. (1998). The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa->luteal transition. Cell Growth Differ 9: 787–794.
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L et al. (2007). Restoration of p53 function leads to tumour regression in vivo. Nature 445: 661–665.
Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D et al. (2002). Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 8: 1136–1144.
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V et al. (2007). Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445: 656–660.
You M, Candrian U, Maronpot RR, Stoner GD, Anderson MW . (1989). Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci USA 86: 3070–3074.
Yuan Y, Qin L, Liu D, Wu RC, Mussi P, Zhou S et al. (2007). Genetic screening reveals an essential role of p27kip1 in restriction of breast cancer progression. Cancer Res 67: 8032–8042.
Zindy F, Cunningham JJ, Sherr CJ, Jogal S, Smeyne RJ, Roussel MF . (1999). Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin-dependent kinases. Proc Natl Acad Sci USA 96: 13462–13467.
Acknowledgements
This work was funded by a NIH T32 CA8046 Interdisciplinary Training Grant in Cancer Research (KSK-S), and an ACS Research Scholar Award and a NIH R01 Research Grant (CJK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelly-Spratt, K., Philipp-Staheli, J., Gurley, K. et al. Inhibition of PI-3K restores nuclear p27Kip1 expression in a mouse model of Kras-driven lung cancer. Oncogene 28, 3652–3662 (2009). https://doi.org/10.1038/onc.2009.226
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.226
Keywords
This article is cited by
-
Checkpoint Kinase 1 Expression Predicts Poor Prognosis in Nigerian Breast Cancer Patients
Molecular Diagnosis & Therapy (2018)
-
FOXO transcription factors in cancer development and therapy
Cellular and Molecular Life Sciences (2016)
-
Sphingomyelin Synthase 1 Regulates Neuro-2a Cell Proliferation and Cell Cycle Progression Through Modulation of p27 Expression and Akt Signaling
Molecular Neurobiology (2015)
-
ARF inhibits the growth and malignant progression of non-small-cell lung carcinoma
Oncogene (2014)
-
Preclinical modeling of EGFR-specific antibody resistance: oncogenic and immune-associated escape mechanisms
Oncogene (2014)