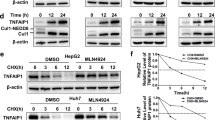
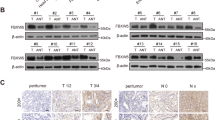
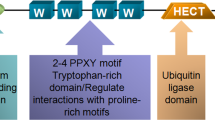
Abstract
Ubiquitin C-terminal hydrolase-L1 (UCH-L1) catalyses the hydrolysis of ubiquitin ester and amide mainly in neuronal cells. Recently it was proposed as a marker with a potential role in carcinogenesis. However, the molecular mechanism underlying the biological function of UCH-L1 in tumor cells is poorly understood. We found that UCH-L1 is highly expressed in non-small lung cancer cell line H157, having high invasive potential, and that the expression of UCH-L1 in tumor cells enhances their invasive potential in vitro and in vivo. UCH-L1 changes cell morphology by regulating cell adhesion through Akt-mediated pathway. Suppressing UCH-L1 expression by RNAi significantly suppressed the invasion in vitro and in vivo, and the activation of Akt and downstream mitogen activated protein kinases c-Jun N-terminal kinases and p38, but not ERK. In Akt-negative mutants, overexpression of UCH-L1 does not affect the invasion and migration capability of H157 cells. These results suggest that UCH-L1 is a key molecule to regulate tumor-cell invasion by upstream activation of Akt.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Bittencourt Rosas SL, Caballero OL, Dong SM, da Costa Carvalho Mda G, Sidransky D, Jen J . (2001). Methylation status in the promoter region of the human PGP9.5 gene in cancer and normal tissues. Cancer Lett 170: 73–79.
Brichory F, Beer D, Le Naour F, Giordano T, Hanash S . (2001). Proteomics-based identification of protein gene product 9.5 as a tumor antigen that induces a humoral immune response in lung cancer. Cancer Res 61: 7908–7912.
Caballero OL, Resto V, Patturajan M, Meerzaman D, Guo MZ, Engles J et al. (2002). Interaction and colocalization of PGP9.5 with JAB1 and p27(Kip1). Oncogene 21: 3003–3010.
Diebold BA, Fowler B, Lu J, Dinauer MC, Bokoch GM . (2004). Antagonistic cross-talk between Rac and Cdc42 GTPases regulates generation of reactive oxygen species. J Biol Chem 279: 28136–28142.
Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A et al. (2006). Ubiquitin hydrolase UCH-L1 rescues b-amyloid-induced decreases in synaptic function and contextual memory. Cell 126: 775–788.
Hibi K, Westra WH, Borges M, Goodman S, Sidransky D, Jen J . (1999). PGP9.5 as a candidate tumor marker for non-small-cell lung cancer. Am J Pathol 155: 711–715.
Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H et al. (1998). Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol 140: 1383–1393.
Huang C, Jacobson K, Schaller MD . (2004). A role for JNK-paxillin signaling in cell migration. Cell Cycle 3: 4–6.
Kim YM, Seo J, Kim YH, Jeong J, Joo HJ, Lee DH et al. (2007). Systemic analysis of tyrosine phosphorylated proteins in angiopoietin-1 induced signaling pathway of endothelial cells. J Proteome Res 6: 3278–3290.
Kurisu S, Suetsugu S, Yamazaki D, Yamaguchi H, Takenawa T . (2005). Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene 24: 1309–1319.
Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury Jr PT . (2002). The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell 111: 209–218.
Liu Y, Lashuel HA, Choi S, Xing X, Case A, Ni J et al. (2003). Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell line. Chem Biol 10: 837–846.
Lowe J, McDermott H, Landon M, Mayer RJ, Wilkinson KD . (1990). Ubiquitin carboxyl-terminal hydrolase (PGP 9.5) is selectively present in ubiquitinated inclusion bodies characteristic of human neurodegenerative diseases. J Pathol 161: 153–160.
Osaka H, Wang YL, Takada K, Takizawa S, Setsuie R, Li H et al. (2003). Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet 12: 1945–1958.
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P et al. (1994). Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78: 59–66.
Reddy KB, Nabha SM, Atanaskova N . (2003). Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev 22: 395–403.
Saigoh K, Wang YL, Suh JG, Yamanishi T, Sakai Y, Kiyosawa H et al. (1999). Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat Genet 23: 47–51.
Song EJ, Yim SH, Kim E, Kim NS, Lee KJ . (2005). Human Fas-associated factor 1, interacting with ubiquitinated proteins and valosin-containing protein, is involved in the ubiquitin-proteasome pathway. Mol Cell Biol 25: 2511–2524.
Tezel E, Hibi K, Nagasaka T, Nakao A . (2000). PGP9.5 as a prognostic factor in pancreatic cancer. Clin Cancer Res 6: 4764–4767.
Toyoshima H, Hunter T . (1994). p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78: 67–74.
Wojciak-Stothard B, Tsang LY, Haworth SG . (2005). Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760.
Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE et al. (2006). The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol 26: 6844–6858.
Yamazaki T, Hibi K, Takase T, Tezel E, Nakayama H, Kasai Y et al. (2002). PGP9.5 as a marker for invasive colorectal cancer. Clin Cancer Res 8: 192–195.
Yoeli-Lerner M, Toker A . (2006). Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle 5: 603–605.
Acknowledgements
This study was supported by SK Corp. Korea, KOSEF through the Center for Cell Signaling & Drug Discovery Research (CCS & DDR, R15-2006-002) at Ewha Womans University, by KOSEF Grant FPR05A2-480. Kim HJ, Nam YK, Kim YM, Kim HJ and Lim S were supported by the Brain Korea 21 project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, H., Kim, Y., Lim, S. et al. Ubiquitin C-terminal hydrolase-L1 is a key regulator of tumor cell invasion and metastasis. Oncogene 28, 117–127 (2009). https://doi.org/10.1038/onc.2008.364
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.364
Keywords
This article is cited by
-
UCHL1 as a novel target in breast cancer: emerging insights from cell and chemical biology
British Journal of Cancer (2022)
-
Ubiquitin C-terminal hydrolase-L1 expression in non-small-cell lung cancer and its association with clinicopathological features and prognosis
Virchows Archiv (2022)
-
Blockage of UCHL1 activity attenuates cardiac remodeling in spontaneously hypertensive rats
Hypertension Research (2020)
-
Downregulated UCHL1 Accelerates Gentamicin-Induced Auditory Cell Death via Autophagy
Molecular Neurobiology (2019)
-
Deubiquitylating enzymes and drug discovery: emerging opportunities
Nature Reviews Drug Discovery (2018)